Rotation of the F1 γ Subunit, Driven by Proton Movement Through F0, Powers ATP Synthesis
Each of the three β subunits in the globular F1 subcomplex of F0F1 can bind ADP and Pi and catalyze the endergonic synthesis of ATP when coupled to the flow of protons from the exoplasmic medium (the intermembrane space in the mitochondrion) to the cytosolic (matrix) medium. However, the energetic coupling of proton flow and ATP synthesis does not take place in the same portions of the protein, because the nucleotide-binding sites on the β subunits of F1, where ATP synthesis occurs, are 9–10 nm from the surface of the membrane-embedded portion of F0 through which the protons flow. The most widely accepted model for ATP synthesis by the F0F1 complex—the binding-change mechanism—posits an indirect coupling (Figure 12-32).
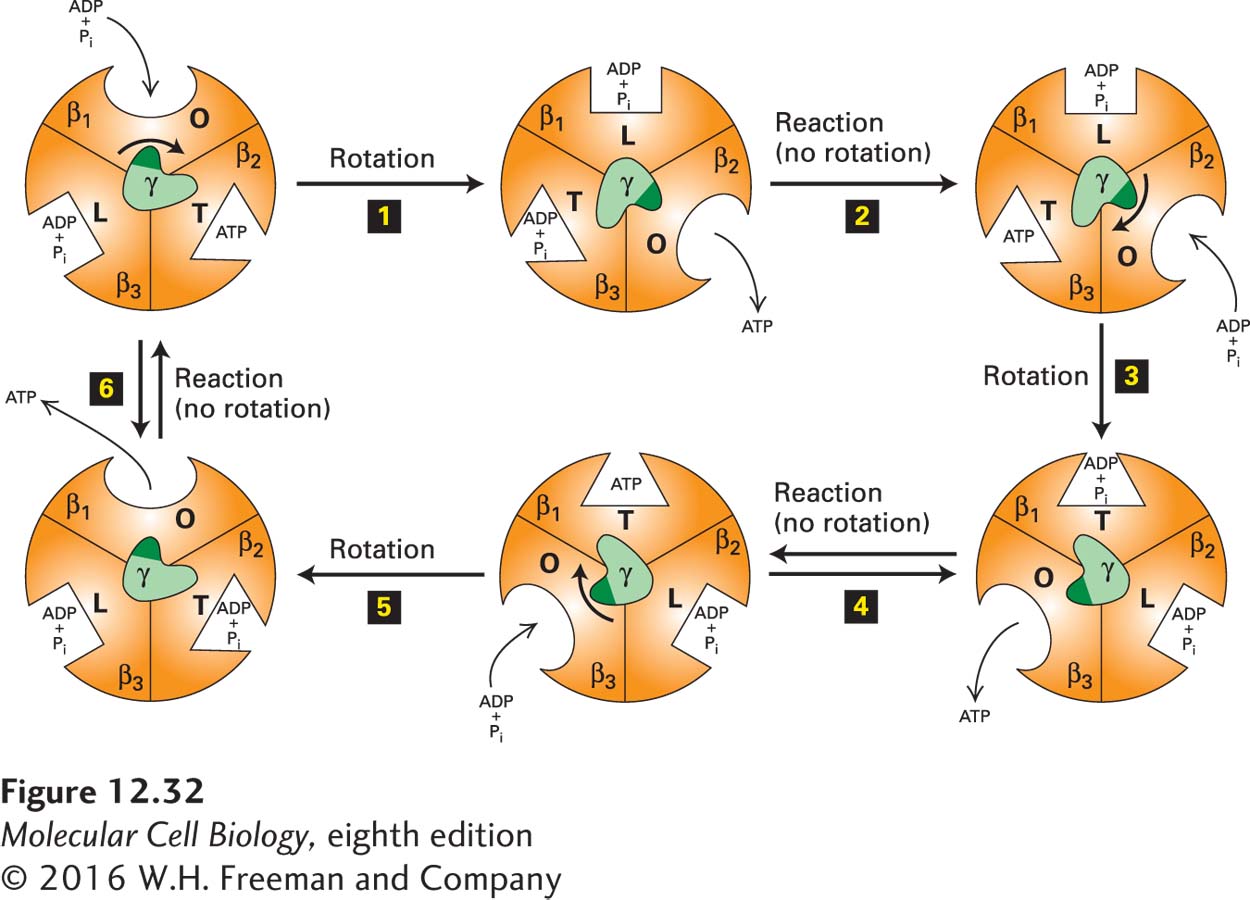
FIGURE 12-32 The binding-change mechanism of ATP synthesis from ADP and Pi. This view is looking up at F1 from the membrane surface (see Figure 12-31). As the γ subunit rotates by 120° in the center, each of the otherwise identical F1 β subunits alternates between three conformational states (O, open, with oval representation of the binding site; L, loose, with a rectangular binding site; T, tight, with a triangular site) that differ in their binding affinities for ATP, ADP, and Pi. The cycle begins (upper left) when ADP and Pi bind loosely to one of the three β subunits (here, arbitrarily designated β1) whose nucleotide-binding site is in the O (open) conformation. Proton flux through the F0 portion of the protein powers a 120° rotation of the γ subunit (relative to the fixed β subunits) (step 1). This causes the rotating γ subunit, which is asymmetric, to push differentially against the β subunits, resulting in a conformational change and an increase in the binding affinity of the β1 subunit for ADP and Pi (O → L), an increase in the binding affinity of the β3 subunit for ADP and Pi that were previously bound (L → T), and a decrease in the binding affinity of the β2 subunit for a previously bound ATP (T → O), causing release of the bound ATP. Step 2: Without additional rotation, the ADP and Pi in the T site (here, in the β3 subunit) form ATP, a reaction that does not require an input of additional energy due to the special environment in the active site of the T state. At the same time, a new ADP and Pi bind loosely to the unoccupied O site on β2. Step 3: Proton flux powers another 120° rotation of the γ subunit, consequent conformational changes in the binding sites (L → T, O → L, T → O), and release of ATP from β3. Step 4: Without additional rotation, the ADP and Pi in the T site of β1 form ATP, and additional ADP and Pi bind to the unoccupied O site on β3. The process continues with rotation (step 5) and ATP formation (step 6) until the cycle is complete, with three ATPs having been produced for every 360° rotation of γ. See P. Boyer, 1989, FASEB J. 3:2164; Y. Zhou et al., 1997, Proc. Natl. Acad. Sci. USA 94:10583; and M. Yoshida, E. Muneyuki, and T. Hisabori, 2001, Nat. Rev. Mol. Cell Biol. 2:669.
According to this mechanism, energy released by the “downhill” movement of protons through F0 directly powers rotation of the c-subunit ring together with its attached γ and ε subunits (see Figure 12-31a). The γ subunit acts as a cam, or nonsymmetrical rotating shaft, whose c ring–driven rotation within the center of the static (αβ)3 hexamer of F1 causes it to push sequentially against each of the β subunits and thus cause cyclical changes in their conformations between three different states. As schematically depicted in a view of the bottom of the (αβ)3 hexamer’s globular structure in Figure 12-32, rotation of the γ subunit relative to the fixed (αβ)3 hexamer causes the nucleotide-binding site of each β subunit to cycle through three conformational states in the following order:
An O (open) state that binds ATP very poorly and ADP and Pi weakly
An L (loose) state that binds ADP and Pi more strongly but cannot bind ATP
A T (tight) state that binds ADP and Pi so tightly that they spontaneously react and form ATP
In the T state, the ATP produced is bound so tightly that it cannot readily dissociate from the site—it is trapped until another rotation of the γ subunit returns that β subunit to the O state, thereby releasing ATP and beginning the cycle again. ATP or ADP also binds to regulatory or allosteric sites on the three α subunits; this binding modifies the rate of ATP synthesis according to the level of ATP and ADP in the matrix, but is not directly involved in the catalytic step that synthesizes ATP from ADP and Pi.
Several types of evidence support the binding-change mechanism. First, biochemical studies showed that on isolated F1 particles, one of the three β subunits can tightly bind ADP and Pi and then form ATP, which remains tightly bound. The measured ΔG for this reaction is near zero, indicating that once ADP and Pi are bound to the T state of a β subunit, they spontaneously form ATP. Importantly, dissociation of the bound ATP from the β subunit on isolated F1 particles occurs extremely slowly. This finding suggested that dissociation of ATP would have to be powered by a conformational change in the β subunit, which in turn would be due to c ring rotation caused by proton movement.
X-ray crystallographic analysis of the (αβ)3 hexamer yielded a striking conclusion: although the three β subunits are identical in sequence and overall structure, the ADP/ATP-binding sites have different conformations in each subunit. The most reasonable conclusion was that the three β subunits cycle in an energy-dependent reaction between three conformational states (O, L, T), in which the nucleotide-binding site has substantially different structures.
In other studies, intact F0F1 complexes were treated with chemical cross-linking agents that covalently linked the γ and ε subunits and the c-subunit ring. The observation that such treated complexes could synthesize ATP or use ATP to power proton pumping indicates that the cross-linked proteins normally rotate together.
Finally, rotation of the γ subunit relative to the fixed (αβ)3 hexamer, as proposed in the binding-change mechanism, was observed directly in the clever experiment depicted in Figure 12-33. In one modification of this experiment in which tiny gold particles, rather than an actin filament, were attached to the γ subunit, rotation rates of 134 revolutions per second were observed. Hydrolysis of three ATPs, which you recall is the reverse reaction catalyzed by the same enzyme, is thought to power one revolution; this result is close to the experimentally determined rate of ATP hydrolysis by F0F1 complexes: about 400 ATPs per second. In a related experiment, a γ subunit linked to an ε subunit and a ring of c subunits was seen to rotate relative to the fixed (αβ)3 hexamer. Rotation of the γ subunit in these experiments was powered by ATP hydrolysis. These observations established that the γ subunit, along with the attached c ring and ε subunit, does indeed rotate, thereby driving the conformational changes in the β subunits that are required for the binding of ADP and Pi, followed by synthesis and subsequent release of ATP.
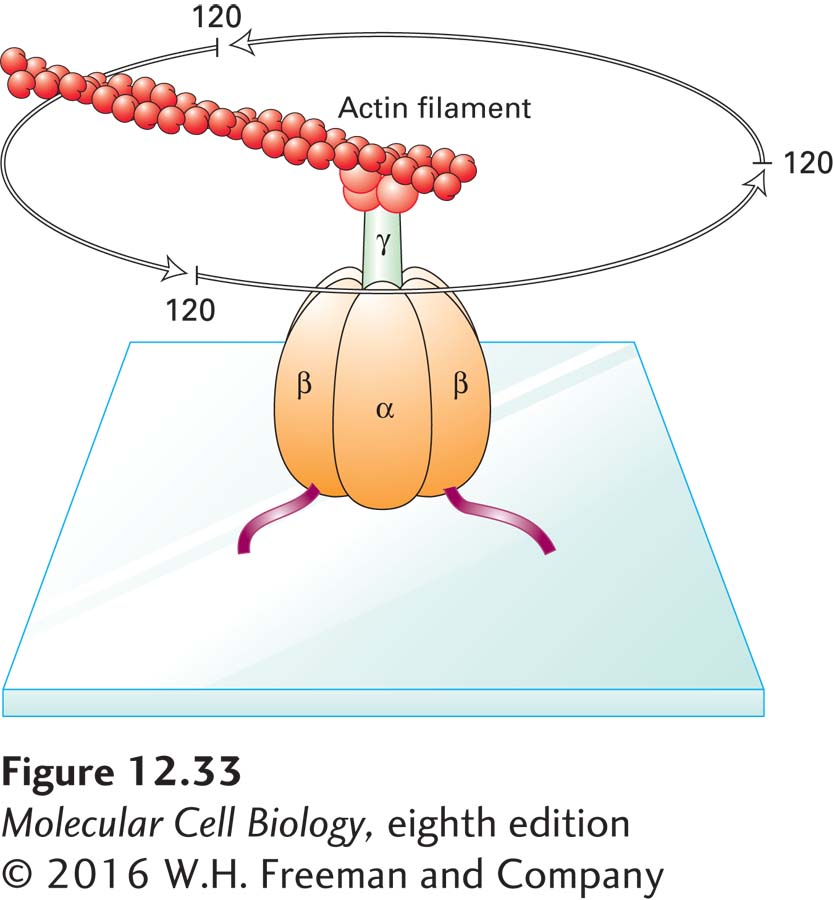
EXPERIMENTAL FIGURE 12-33 The γ subunit of the F1 subcomplex rotates relative to the (αβ)3 hexamer. F1 subcomplexes were engineered to contain β subunits with an additional His-6 sequence, which causes them to adhere to a glass plate coated with a metal reagent that binds polyhistidine. The γ subunit in the engineered F1 subcomplexes was linked covalently to a fluorescently labeled actin filament. When viewed in a fluorescence microscope, the actin filament was seen to rotate counterclockwise in discrete 120° steps in the presence of ATP due to ATP hydrolysis by the β subunits. See H. Noji et al., 1997, Nature 386:299, and R. Yasuda et al., 1998, Cell 93:1117.

