F0 c Ring Rotation Is Driven by Protons Flowing Through Transmembrane Channels
Each copy of the c subunit contains two membrane-spanning α helices that form a hairpin-like structure. An aspartate residue, Asp-61 (E. coli ATPase numbering), in the center of one of these helices in each c subunit is thought to play a key role in proton movement by binding and releasing protons as they traverse the membrane. Chemical modification of this aspartate by the poison dicyclohexylcarbodiimide, or its mutation to alanine, specifically blocks proton movement through F0. According to one current model, the protons traverse the membrane via two staggered half-channels, I and II (see Figure 12-31a and b). They are called half-channels because each extends only halfway across the membrane; the intramembrane termini of the channels are at the level of Asp-61 in the middle of the membrane. Half-channel I is open only to the exoplasmic face, and half-channel II is open only to the cytosolic face. Prior to rotation, each of the Asp-61 carboxylate side chains in the c subunits is bound to a proton, except that on the c subunit in contact with half-channel I. The negative charge on that unprotonated carboxylate (the “empty” proton-binding site; see Figure 12-31b, bottom) is neutralized by interaction with the positively charged side chain of Arg-210 from the a subunit. Proton translocation across the membrane begins when a proton from the exoplasmic medium moves upward through half-channel I (Figure 12-31b, step 1). As that proton moves into the empty proton-binding site, it displaces the Arg-210 side chain, which swings toward the filled proton-binding site of the adjacent c subunit in contact with half-channel II (step 2). As a consequence, the positive side chain of Arg-210 displaces the proton bound to Asp-61 of the adjacent c subunit. This displaced proton is now free to travel up half-channel II and out into the cytosolic medium (step 3). Thus when one proton entering from half-channel I binds to the c ring, a different proton is released to the opposite side of the membrane via half-channel II. Rotation of the entire c ring due to thermal/Brownian motion (step 4) then allows the newly unprotonated c subunit to move into alignment above half-channel I as an adjacent, protonated c subunit rotates in to take its place under half-channel II. The entire cycle is then repeated (step 5) as additional protons move down their electrochemical gradient from the exoplasmic medium to the cytosolic medium. During each partial rotation (360° divided by the number of c subunits in the ring), the c ring rotation is ratcheted, in that net movement of the ring occurs in only one direction. The energy driving the protons across the membrane, and thus the rotation of the c ring, comes from the electrochemical gradient across the membrane. If the direction of proton flow is reversed, which can be done by experimentally reversing the direction of the proton gradient and the proton-motive force, the direction of c ring rotation is reversed.
Because the γ subunit of F1 is tightly attached to the c ring of F0, rotation of the c ring associated with proton movement causes rotation of the γ subunit. According to the binding-change mechanism, a 120° rotation of γ powers synthesis of one ATP (see Figure 12-32). Thus complete rotation of the c ring by 360° would generate three ATPs. In E. coli, where the F0 composition is a1b2c10, movement of 10 protons drives one complete rotation and thus synthesis of three ATPs. This value is consistent with experimental data on proton flux during ATP synthesis, providing indirect support for the model coupling proton movement to c ring rotation depicted in Figure 12-31. The F0 from chloroplasts contains 14 c subunits per ring, and movement of 14 protons would be needed for synthesis of three ATPs. Why these otherwise similar F0F1 complexes have evolved to have different H+:ATP ratios is not clear.
High-resolution electron microscopic tomography (Figure 12-34) has provided additional insights into the structure of the c ring/a subunit interface and other features of F0F1 structure and function. The experiments were performed using F0F1 either dissolved in detergent, then incorporated into artificial phospholipid bilayers, or in isolated mitochondrial membranes. Figures 12-34a and b show two views of the two membrane-spanning α helices in each copy of the c subunit (green) that together form the c ring. In a portion of the a subunit (orange), a bundle of four α helices that are almost parallel to and embedded within the inner mitochondrial membrane forms the interface with the c ring and positions the side chain of Arg 210 adjacent to the c ring so that it can mediate proton displacement from Asp 61 as shown in Figure 12-31. The c ring/a subunit interface also forms the two proton half-channels through which protons flow out of the intermembrane space (red arrow), around the c ring (black arrows in Figure 12-34b), and then out into the matrix (red arrow). Each F0F1 monomer bends the membrane by approximately 43° (Figure 12-34c). The monomers dimerize to impart high membrane curvature (~86°) and then align in long rows, contributing to the formation of the edges and tips of the pancake-like (flat) and tubular cristae (Figure 12-34d).
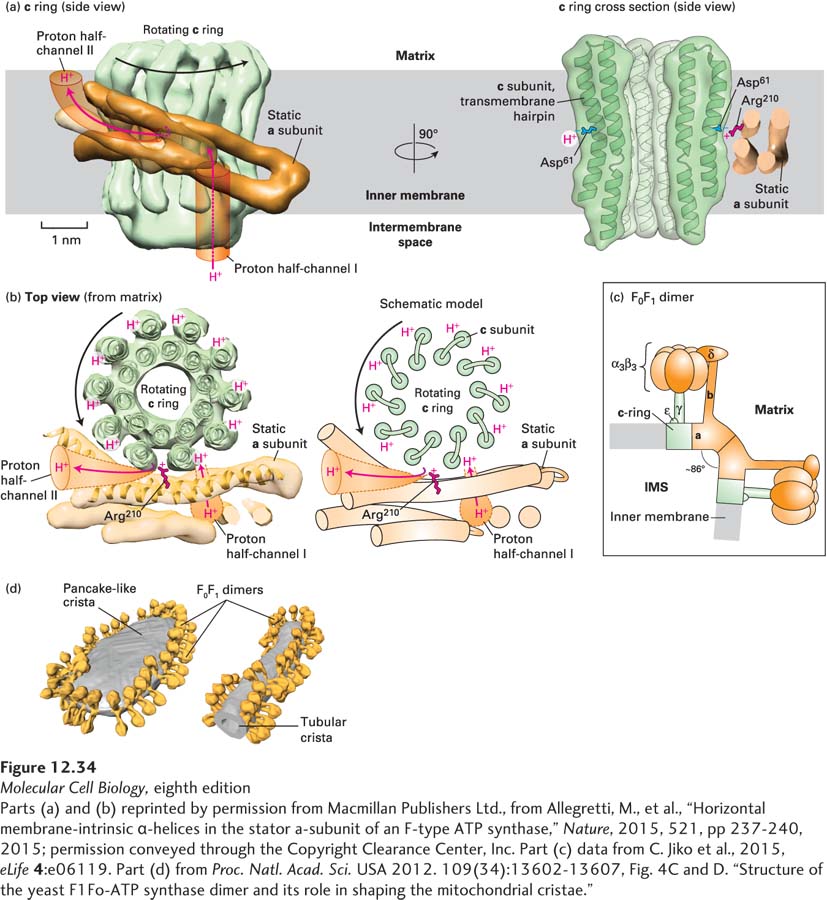
EXPERIMENTAL FIGURE 12-34 High-resolution electron microscopy-based mechanism of proton translocation and bending of cristae membranes by ATP synthase. (a) and (b) The interface between the c ring (green) and a subunit (orange) of detergent-solubilized mitochondrial ATP synthase from the alga Polytomella sp., imaged by single-particle cryoelectron microscopy (~0.62 nm resolution), is shown (a) from within the plane of the inner mitochondrial membrane (side view) and (b) after a 90° rotation (top view). The movement of protons through half-channels I and II and the rotation of the c ring are described in detail in Figure 12-31. (a) Cross section through the c ring (right) shows that each c subunit is a transmembrane helical hairpin – two adjacent transmembrane α helices connected by a short nonhelical linker on the matrix side of the membrane. The negative side chain of the c subunit’s Asp61 in the middle of the membrane is thought to both serve as a binding site for translocating protons and interact with the side chain of the a subunit’s Arg210. (c) A model of the bovine heart mitochondrial ATP synthase is based on cryoelectron tomography and electron crystallographic image processing from crystalline ATP synthase in artificial membranes. Each F0F1 monomer bends the membrane by ~43° toward the intermembrane space (IMS), resulting in dimers bending the membrane by ~86°. The rotating c ring and γ and ε subinits are colored green, and the remaining static portions of the enzyme are shown in orange. (d) Cryoelectron tomographic image of frozen membranes from purified Saccharomyces cerevisiae (yeast) mitochondria. The surfaces of the ATP synthase complexes (orange) and the membrane (gray) show that the enzymes dimerize as in (c) and align into long rows that bend the membranes into characteristic tubular and flat, pancake-like cristae.
[Parts (a) and (b) reprinted by permission from Macmillan Publishers Ltd., from Allegretti, M., et al., “Horizontal membrane-intrinsic α-helices in the stator a-subunit of an F-type ATP synthase,” Nature, 2015, 521, pp 237-240, 2015; permission conveyed through the Copyright Clearance Center, Inc. Part (c) data from C. Jiko et al., 2015, eLife 4:e06119. Part (d) from Proc. Natl. Acad. Sci. USA 2012. 109(34):13602-13607, Fig. 4C and D. “Structure of the yeast F1Fo-ATP synthase dimer and its role in shaping the mitochondrial cristae.”]
