Photosystems Comprise a Reaction Center and Associated Light-Harvesting Complexes
The absorption of light energy and its conversion into chemical energy occurs in multiprotein complexes called photosystems. Found in all photosynthetic organisms, both eukaryotic and prokaryotic, photosystems consist of two closely linked components: a reaction center, where the primary events of photosynthesis—light absorption and generation of high-energy electrons—occur; and an antenna complex consisting of numerous protein complexes, including internal antenna proteins. Each photosystem is also associated with external antenna complexes termed light-harvesting complexes (LHCs), made up of specialized proteins that capture light energy and efficiently transmit it to the reaction center to generate high-energy electrons (see Figure 12-38).
Both reaction centers and antennas contain tightly bound light-absorbing pigment molecules. Chlorophyll a, the principal pigment involved in photosynthesis, is present in both reaction centers and antennas. In addition to chlorophyll a, antennas contain other light-absorbing pigments: chlorophyll b in vascular plants and carotenoids in both plants and photosynthetic bacteria. Carotenoids consist of long branched hydrocarbon chains with alternating single and double bonds; they are similar in structure to the visual pigment retinal (see Figure 15-19), which absorbs light in the eye. The presence of various antenna pigments, which absorb light at different wavelengths, greatly extends the range of light that can be absorbed and used for photosynthesis.
One of the strongest pieces of evidence for the involvement of chlorophylls and carotenoids in photosynthesis is that the absorption spectrum of these pigments is similar to the action spectrum of photosynthesis (Figure 12-40). The latter is a measure of the relative ability of light of different wavelengths to support photosynthesis.
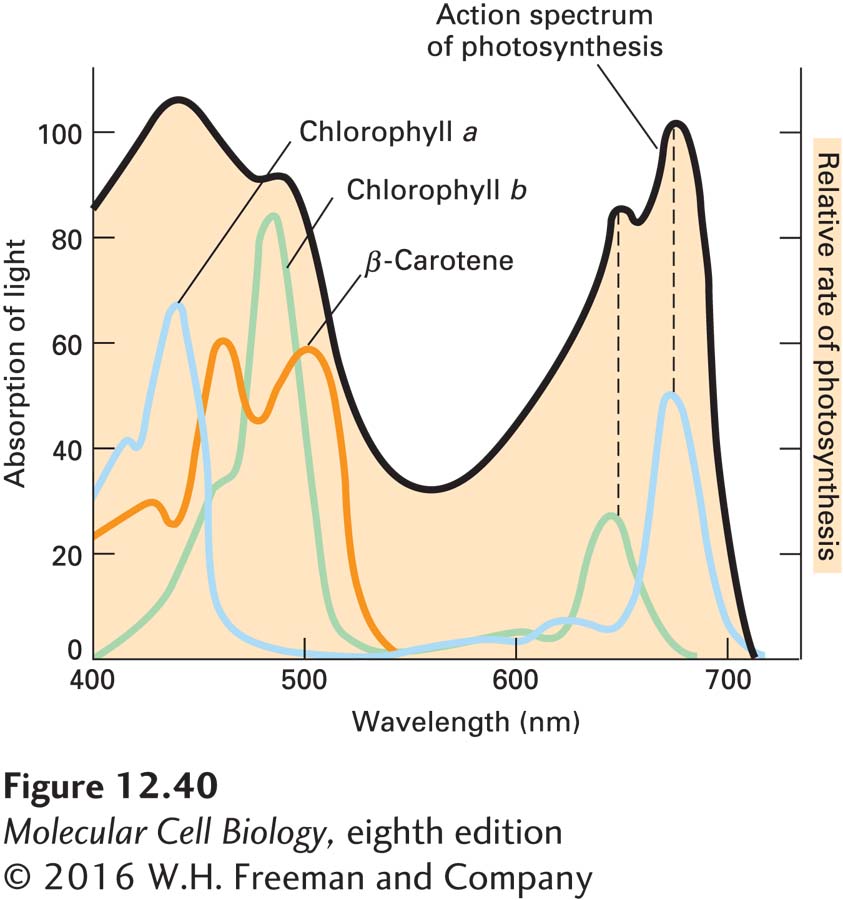
EXPERIMENTAL FIGURE 12-40 The rate of photosynthesis is greatest at the wavelengths of light absorbed by three plant pigments. The action spectrum of photosynthesis in plants (the relative ability of light of different wavelengths to support photosynthesis) is shown in black. The energy from light can be converted into ATP only if it can be absorbed by pigments in the chloroplast. Absorption spectra (showing how well light of different wavelengths is absorbed) for three photosynthetic pigments present in the antennas of plant photosystems are shown in color. Comparison of the action spectrum of photosynthesis with the individual absorption spectra of these pigments suggests that photosynthesis at 680 nm is primarily due to light absorbed by chlorophyll a; at 650 nm, to light absorbed by chlorophyll b; and at shorter wavelengths, to light absorbed by chlorophylls a and b and by carotenoid pigments, including β-carotene.
When chlorophyll a (or any other molecule) absorbs visible light, the absorbed light energy raises electrons in the chlorophyll a to a higher-energy (excited) state. This state differs from the ground (unexcited) state largely in the distribution of the electrons around the C and N atoms of the porphyrin ring. Excited states are unstable, and the electrons return to the ground state by one of several competing processes. For chlorophyll a molecules dissolved in organic solvents such as ethanol, the principal reactions that dissipate the excited-state energy are the emission of light (fluorescence and phosphorescence) and thermal emission (heat). However, when the same chlorophyll a is bound in the unique protein environment of the reaction center, dissipation of excited-state energy occurs by a different process, which is the key to photosynthesis.
