Photoelectron Transport from Energized Reaction-Center Chlorophyll a Produces a Charge Separation
Within the reaction center, two adjacent chlorophyll a molecules, referred to as the special-pair chlorophylls, lie close to the luminal face of the thylakoid membrane (Figure 12-41). When a photon of light with a wavelength of about 680 nm is absorbed by one of these two chlorophyll a molecules, the energy of that chlorophyll a molecule increases by 42 kcal/mol (the first excited state). This energized molecule rapidly donates an electron to the adjacent chlorophyll, which passes it on to a series of intermediate acceptors. In this manner, the electron is rapidly passed on to the primary electron acceptor, quinone Q, near the stromal surface of the thylakoid membrane. This light-driven electron transfer, called photoelectron transport, depends on the unique environment of both the chlorophylls and the acceptor within the reaction center. Photoelectron transport, which occurs nearly every time a photon is absorbed, leaves a positive charge on the chlorophyll a close to the luminal surface of the thylakoid membrane (on the opposite side from the stroma) and generates a reduced, negatively charged acceptor (Q−) near the stromal surface.
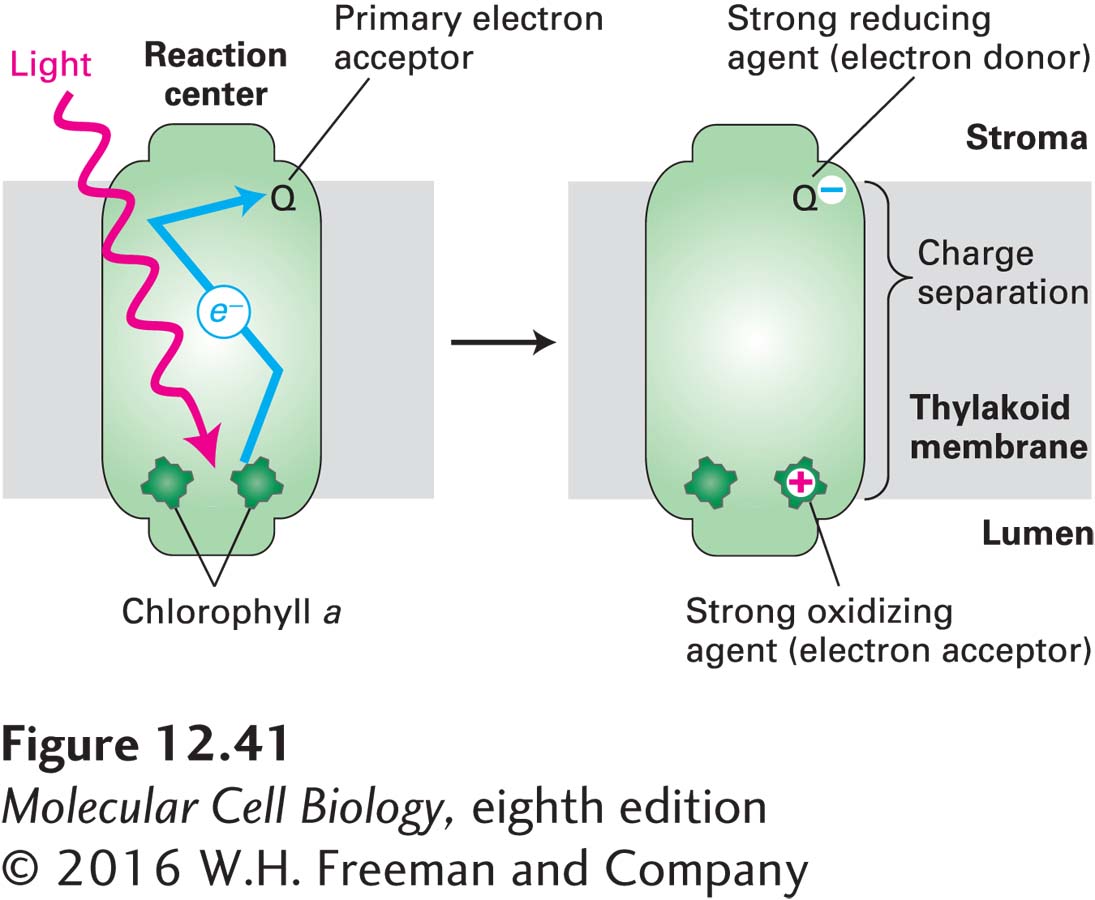
FIGURE 12-41 Photoelectron transport, the primary event in photosynthesis. After absorption of a photon of light, one of the excited special-pair chlorophyll a molecules in the reaction center (left) donates, via several intermediates (not shown), an electron to a loosely bound acceptor molecule, the quinone Q, on the stromal surface of the thylakoid membrane, creating an essentially irreversible charge separation across the membrane (right). Subsequent transfers of this electron release energy that is used to generate ATP and NADPH (see Figures 12-43 and 12-44). The positively charged chlorophyll a+ generated when the light-excited electron moves to Q is eventually neutralized by the transfer to the chlorophyll a+ of another electron. In plants, the oxidation of H2O to O2 provides this neutralizing electron and takes place in a multiprotein complex called photosystem II (see Figure 12-44). Photosystem I uses a similar photoelectron transport pathway, but instead of oxidizing water, it receives an electron from a protein carrier called plastocyanin to neutralize the positive charge on chlorophyll a+ (see Figure 12-44).
The Q− produced by photoelectron transport is a powerful reducing agent with a strong tendency to transfer an electron to another molecule, ultimately to NADP+. The positively charged chlorophyll a+, a strong oxidizing agent, attracts an electron from an electron donor on the luminal surface to regenerate the original chlorophyll a. In plants, the oxidizing power of four chlorophyll a+ molecules is used, by way of intermediates, to remove four electrons from two H2O molecules bound to a site on the luminal surface to form O2:
2 H2O + 4 chlorophyll a+ → 4 H+ + O2 + 4 chlorophyll a
These potent biological reductants and oxidants provide all the energy needed to drive all subsequent reactions of photosynthesis: electron transport (stage 2), ATP synthesis (stage 3), and CO2 fixation (stage 4).
Chlorophyll a also absorbs light at discrete wavelengths shorter (and therefore of higher energy) than 680 nm (see Figure 12-40). Such absorption raises the molecule into one of several excited states whose energies are higher than that of the first excited state described above, and which decay by releasing energy within 2 × 10−12 seconds (2 picoseconds, ps) to the lower-energy first excited state, with loss of the extra energy as heat. Because photoelectron transport and the resulting charge separation occur only from the first excited state of the reaction-center chlorophyll a, the quantum yield—the amount of photosynthesis per absorbed photon—is the same for all wavelengths of visible light shorter than 680 nm. How closely the wavelength of light matches the absorption spectrum of the pigment determines how likely it is that the photon will be absorbed. Once absorbed, the photon’s exact wavelength is not critical, provided it is at least energetic enough to push the chlorophyll a into the first excited state.
