The Single Photosystem of Purple Bacteria Generates a Proton-Motive Force but No O2
The mechanism of charge separation in the photosystem located in the plasma membrane of purple bacteria (Figure 12-43) is identical to that in plants outlined earlier. The reaction-center protein contains the prosthetic groups that absorb light and transport electrons during photosynthesis. These prosthetic groups include “special-pair” bacteriochlorophyll a molecules equivalent to the reaction-center chlorophyll a molecules in plants, as well as several other pigments and two quinones, termed QA and QB, that are structurally similar to mitochondrial ubiquinone. The energy from absorbed light is used to strip an electron from a reaction-center bacteriochlorophyll a molecule and transfer it to QA via several different pigments in a series of very rapid reactions taking between 4 and 200 ps. Then, in the slowest step, in about 200 microseconds (µs), the electron moves from QA to the primary electron acceptor QB, which is loosely bound to a site on the cytosolic membrane face. The chlorophyll thereby acquires a positive charge, and QB acquires a negative charge.
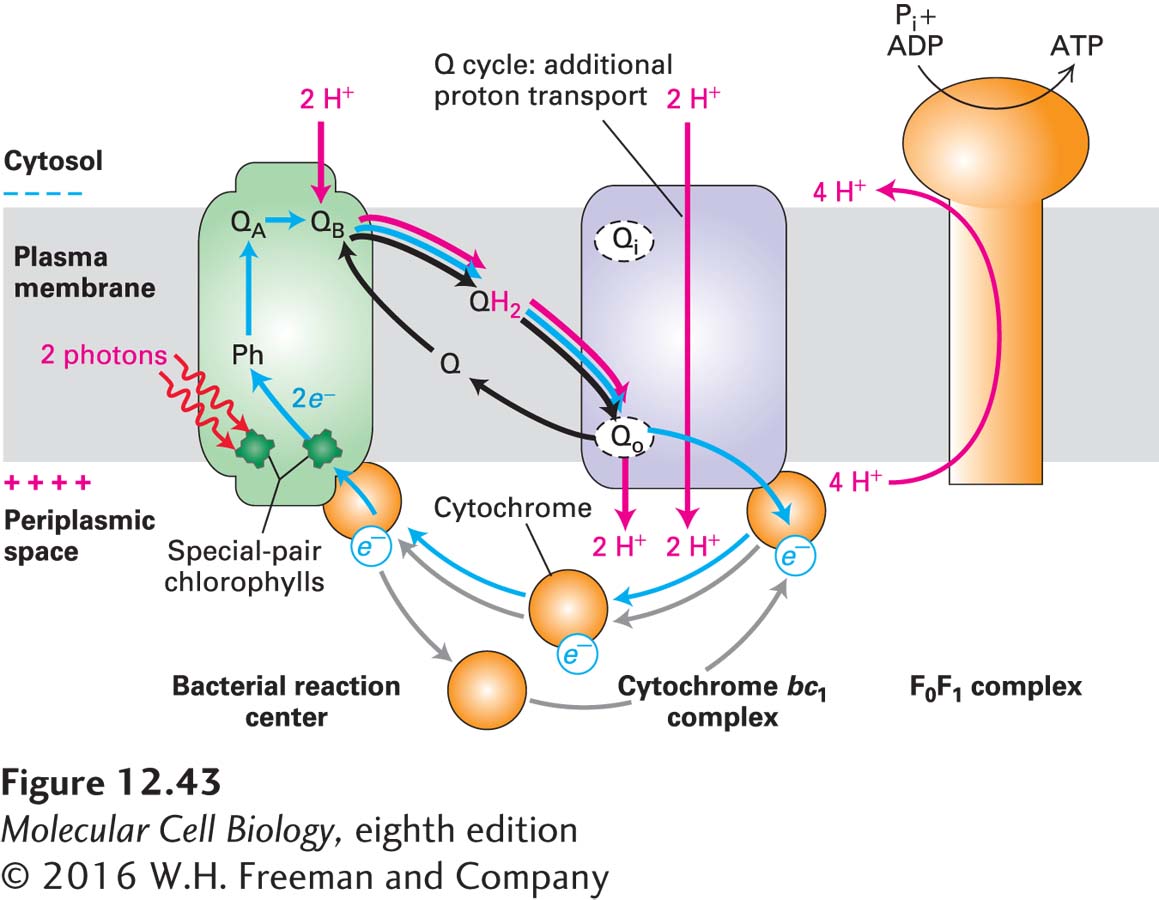
FIGURE 12-43 Cyclic electron flow in the single photosystem of purple bacteria. Cyclic electron flow generates a proton-motive force but no O2. Blue arrows indicate flow of electrons; red arrows indicate proton movement. (Left) Energy absorbed directly from light or funneled from an associated LHC (not illustrated here) energizes one of the special-pair chlorophylls in the reaction center. Photoelectron transport from the energized chlorophyll, via an accessory chlorophyll, pheophytin (Ph), and quinone A (QA), to quinone B (QB), which forms the semiquinone Q•− and leaves a positive charge on the chlorophyll. Following absorption of a second photon and transfer of a second electron to the semiquinone, the quinone rapidly picks up two protons from the cytosol to form QH2. (Center) After diffusing through the membrane and binding to the Qo site on the periplasmic (exoplasmic) face of the cytochrome bc1 complex, QH2 donates two electrons and simultaneously gives up two protons to the external medium in the periplasmic space, generating a proton electrochemical gradient (proton-motive force) that drives ATP synthesis (right). Electrons are transported back to a reaction-center chlorophyll via a soluble cytochrome, which diffuses in the periplasmic space. Note the cyclical path (blue) of electrons. Operation of a Q cycle in the cytochrome bc1 complex pumps additional protons across the membrane to the external medium, as in mitochondria. See J. Deisenhofer and H. Michael, 1991, Annu. Rev. Cell Biol. 7:1.
After the primary electron acceptor, QB, in the bacterial reaction center accepts one electron, forming QB•−, it accepts a second electron from the same reaction-center chlorophyll following its re-excitation (e.g., by absorption of a second photon or by transfer of energy from antenna molecules). The quinone then binds two protons from the cytosol, forming reduced quinone (QH2), which is released from the reaction center (see Figure 12-43). QH2 diffuses within the bacterial membrane to the Qo site on the exoplasmic face of a cytochrome bc1 electron-transport complex, which is similar in structure to complex III in mitochondria. There it releases its two protons into the periplasmic space (the space between the plasma membrane and the bacterial cell wall), generating a proton-motive force across the plasma membrane. Simultaneously, QH2 releases its two electrons, which move through the cytochrome bc1 complex exactly as depicted for the mitochondrial complex III (CoQH2–cytochrome c reductase) in Figure 12-24. The Q cycle in the bacterial reaction center, like the Q cycle in mitochondria, pumps additional protons from the cytosol to the periplasmic space, thereby increasing the proton-motive force.
The acceptor for electrons transferred from the reaction center and through the cytochrome bc1 complex is a soluble cytochrome, a one-electron carrier, in the periplasmic space, which is reduced from the Fe3+ to the Fe2+ state (see Figure 12-43). The reduced cytochrome (analogous to cytochrome c in mitochondria) then diffuses to a reaction center, where it releases its electron to a positively charged chlorophyll a+, returning that chlorophyll to the uncharged ground state and the cytochrome to the Fe3+ state. This cyclic electron flow generates no oxygen and no reduced coenzymes, but it has generated a proton-motive force.
As in other systems, this proton-motive force is used both by the F0F1 complex located in the bacterial plasma membrane to synthesize ATP and by transport proteins to move molecules across the membrane against a concentration gradient.
