Cyclic Electron Flow Through PSI Generates a Proton-Motive Force but No NADPH or O2
As we’ve seen, electrons from reduced ferredoxin in PSI are transferred to NADP+ during linear electron flow, resulting in production of NADPH (see Figure 12-44). In some circumstances, however, especially when plants are stressed by conditions such as drought, high light intensity, or low carbon dioxide levels, cells must generate greater amounts of ATP relative to NADPH than they can produce by linear electron flow. To do this, they photosynthetically produce ATP from PSI without concomitant NADPH production. This is accomplished by a PSI-dependent and PSII-independent process called cyclic photophosphorylation, or cyclic electron flow (Figure 12-45). In this process, electrons cycle between PSI, ferredoxin, plastoquinone (Q), and the cytochrome bf complex, bypassing the ferredoxin-NADP+ reductase at PSI that normally generates NADPH. Thus, during cyclic electron flow, proton pumping permits additional ATP synthesis, but no net NADPH is generated, and there is no oxidization of H2O to produce O2.
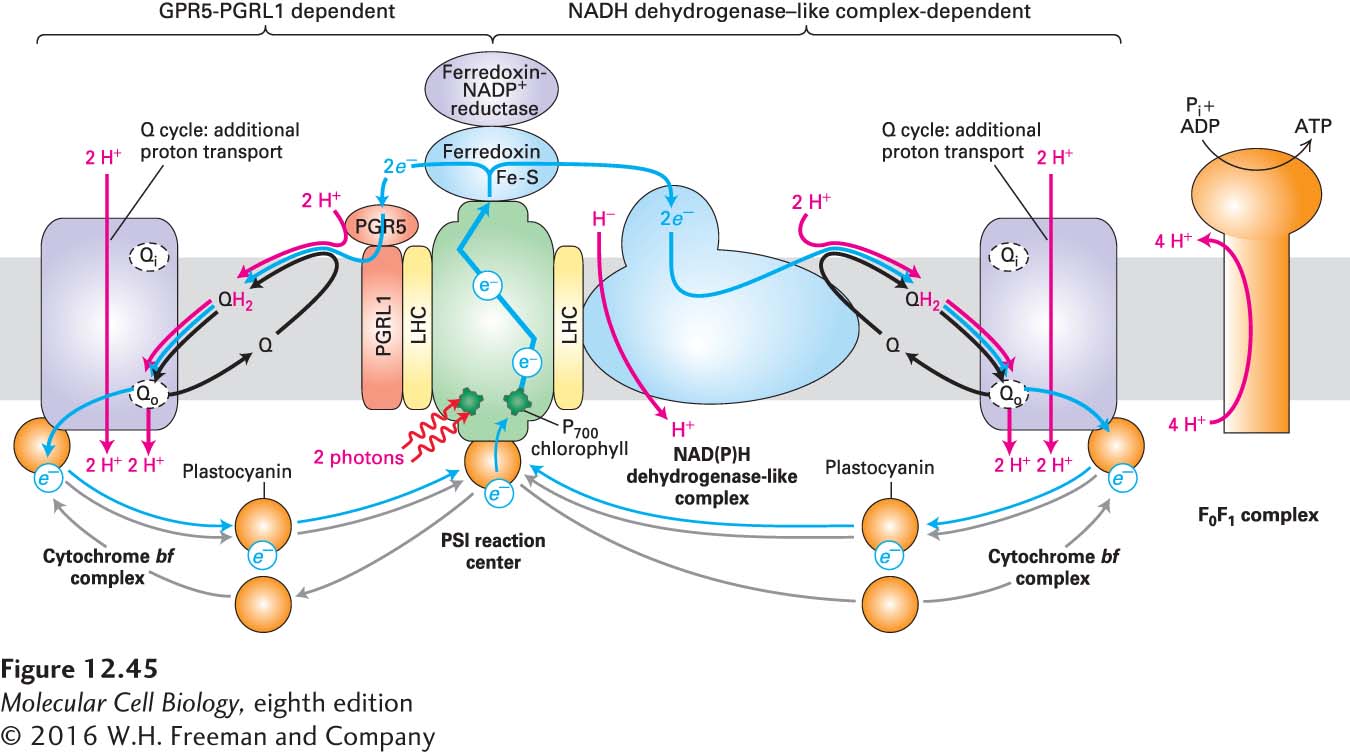
FIGURE 12-45 Cyclic electron flow in plants, which generates a proton-motive force and ATP but no oxygen or NADPH. In cyclic electron flow, light energy is used by PSI to transport electrons in a cycle to generate a proton-motive force and ATP without oxidizing water or generating NADPH. High-energy electrons are transferred via the ferredoxin of PSI either to a PGR5-PGRL1 heterodimer (red, left pathway) or to the NADH dehydrogenase–like complex (blue, right pathway), where they then reduce plastoquinone (Q) to QH2. Each of these two electron acceptors forms independent supercomplexes with PSI via light harvesting complex (LHC) subunits (yellow). (The PGR5-PGRL1 heterodimer and NADH dehydrogenase–like complex are not found together in the same supercomplex, but here are drawn together with only one PSI to emphasize the similarities of the two mechanisms of cyclic electron flow.) QH2 then transfers the electrons to the cytochrome bf complex, then to plastocyanin, and finally back to PSI, as is the case for the linear electron flow pathway (see Figure 12-44).
In higher plants, there are two cyclic electron flow pathways that control the ATP:NADPH ratio (see Figure 12-45). The major pathway is the PGR5-PGRL1-dependent pathway, which we shall describe shortly; this pathway ensures efficient photosynthesis and protects against stress. The minor pathway is the NADH dehydrogenase–like complex-dependent pathway, which appears to respond to stress and to be a target of H2O2-mediated regulation. The NADH dehydrogenase–like complex is a very large multiprotein complex that is very similar in shape and composition to mitochondrial complex I (see Figure 12-22), which oxidizes NADPH or NADH while reducing Q to QH2. The NADH dehydrogenase–like complex, however, appears to lack the subunit necessary for NADPH oxidation.
During cyclic electron flow, high-energy electrons generated by light absorption and photoelectric transport in PSI are transferred either to the PGR5-PGRL1 heterodimer or to the NADH dehydrogenase–like complex from the ferredoxin subunit of PSI. Indeed, there is evidence that each of these two electron acceptors independently associates with PSI in supercomplexes mediated by LHC subunits. Both of these electron acceptors then reduce Q to QH2, which then delivers protons and electrons to the cytochrome bf complex via a Q cycle, as we described earlier for linear electron flow (see Figure 12-44). Protons are transported across the thylakoid membrane into the lumen by the cytochrome bf complex and possibly by the NADH dehydrogenase–like complex. Finally, plastocyanin returns the electrons from the cytochrome bf complex to PSI to complete the cycle. This cyclic electron flow is similar to the cyclical process that occurs in the single photosystem of purple bacteria (see Figure 12-43). The proton-motive force generated by cyclic electron flow drives ATP synthesis by the F0F1 complex (ATP synthase) and thus increases the ATP:NADPH ratio.
