Light and Rubisco Activase Stimulate CO2 Fixation
The Calvin cycle enzymes that catalyze CO2 fixation are rapidly inactivated in the dark, thereby conserving ATP that is generated in the dark (for example, by the breakdown of starch) for other synthetic reactions, such as lipid and amino acid biosynthesis. One mechanism that contributes to this control is the pH dependence of several Calvin cycle enzymes. Because protons are transported from the stroma into the thylakoid lumen during photoelectron transport (see Figure 12-44), the pH of the stroma increases from ~7 in the dark to ~8 in the light. The increased activity of several Calvin cycle enzymes at the higher pH promotes CO2 fixation in the light.
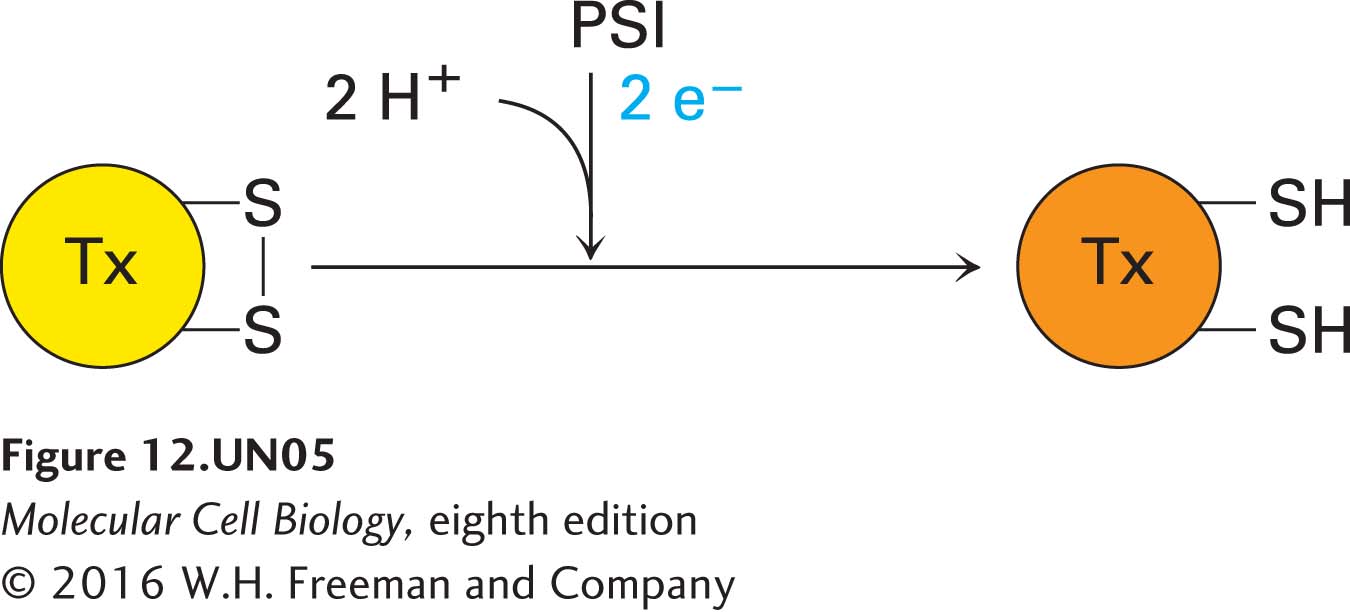
A stromal protein called thioredoxin (Tx) also plays a role in controlling some Calvin cycle enzymes. In the dark, thioredoxin contains a disulfide bond; in the light, electrons are transferred from PSI, via ferredoxin, to thioredoxin, reducing its disulfide bond:
Reduced thioredoxin then activates several Calvin cycle enzymes by reducing disulfide bonds in those enzymes. In the dark, when thioredoxin becomes reoxidized, these enzymes are reoxidized and so inactivated. Thus the activities of these enzymes are sensitive to the redox state of the stroma, which in turn is light sensitive—an elegant mechanism for the regulation of enzymatic activity by light.
Rubisco is one such light/redox-sensitive enzyme, although its regulation is very complex and not yet fully understood. Rubisco is spontaneously activated in the presence of high CO2 and Mg2+ concentrations. The activating reaction entails the covalent addition of CO2 to the side-chain amino group of a lysine in the enzyme’s active site, forming a carbamate group that then binds a Mg2+ ion, which is required for enzymatic activity. Under normal conditions, however, with ambient levels of CO2, this reaction is slow and usually requires catalysis by rubisco activase, a member of the AAA+ family of ATPases. Rubisco activase hydrolyzes ATP and uses the energy released to clear the active site of rubisco so that CO2 can be added to its active site lysine. Rubisco activase also accelerates an activating conformational change in rubisco (from an inactive-closed to an active-opened state). The regulation of rubisco activase by thioredoxin is, at least in part in some species, responsible for rubisco’s light/redox sensitivity. Furthermore, rubisco activase’s activity is sensitive to the ratio of ATP to ADP. If that ratio is low (relatively high ADP), then the activase will not activate rubisco (and so the cell will expend less of its scarce ATP to fix carbon). Photosynthesis is sensitive to a variety of typical plant stresses—moderate heat, cool temperatures, drought (limited water), high salt, high light intensity, and UV radiation. At least some of these stresses influence CO2 fixation by reducing the activity of rubisco activase and thus rubisco. Inhibition of CO2 fixation reduces consumption of NADPH. Under strong light conditions, the high NADPH/NADP+ ratio can reduce electron flow to NADP+ and increase leakage to O2, resulting in increased ROS formation, which can both initiate cellular signaling pathways and interfere with a variety of cellular processes. Given the key role of rubisco in controlling energy utilization and carbon flux—both in individual chloroplasts and, in a sense, throughout the entire biosphere—it is not surprising that its activity is tightly regulated.