Studies with Chimeric Proteins Demonstrate Important Features of Mitochondrial Protein Import
Dramatic evidence for the ability of mitochondrial matrix–targeting sequences to direct the import of proteins was obtained with chimeric proteins produced by recombinant DNA techniques. For example, the matrix-targeting sequence of alcohol dehydrogenase can be fused to the N-terminus of dihydrofolate reductase (DHFR), which normally resides in the cytosol. Cell-free translocation assays show that in the presence of chaperones, which prevent the C-terminal DHFR segment from folding in the cytosol, the chimeric protein is transported into the matrix (Figure 13-25a). The inhibitor methotrexate, which binds tightly to the active site of DHFR and greatly stabilizes its folded conformation, renders the chimeric protein resistant to unfolding by cytosolic chaperones. When translocation assays are performed in the presence of methotrexate, the chimeric protein does not completely enter the matrix. This finding demonstrates that a precursor must be unfolded in order to traverse the import pores in both mitochondrial membranes.
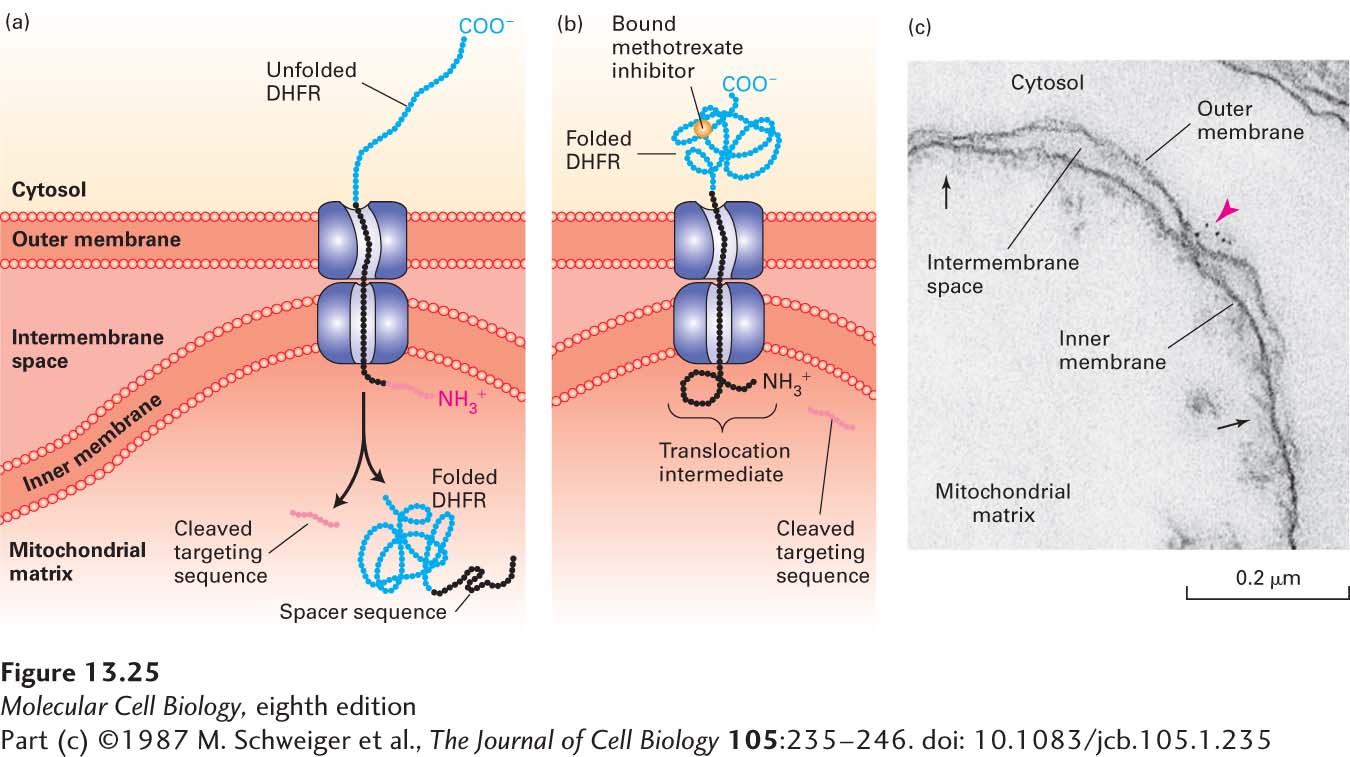
EXPERIMENTAL FIGURE 13-25 Experiments with chimeric proteins elucidate mitochondrial protein import processes. These experiments show that a matrix-targeting sequence alone directs proteins to the mitochondrial matrix and that only unfolded proteins are translocated across both mitochondrial membranes. The chimeric protein in these experiments contained a matrix-targeting signal at its N-terminus (red), followed by a spacer sequence of no particular function (black), and then by dihydrofolate reductase (DHFR), an enzyme normally present only in the cytosol. (a) When the DHFR segment is unfolded, the chimeric protein moves across both membranes to the matrix of an energized mitochondrion, and the matrix-targeting signal is then removed. (b) When the C-terminus of the chimeric protein is locked in the folded state by binding of methotrexate, translocation is blocked. If the spacer sequence is long enough to extend across both transport channels, a stable translocation intermediate, with the targeting sequence cleaved off, is generated in the presence of methotrexate, as shown here. (c) The C-terminus of the translocation intermediate in (b) can be detected by incubating the mitochondria with antibodies that bind to the DHFR segment, followed by gold particles coated with bacterial protein A, which binds nonspecifically to antibody molecules (see Figure 4-33). An electron micrograph of a sectioned sample reveals gold particles (red arrowhead) bound to the translocation intermediate at a contact site between the inner and outer membranes. Other contact sites (black arrows) are also evident. See J. Rassow et al., 1990, FEBS Lett. 275:190.
[Part (c) ©1987 M. Schweiger et al., The Journal of Cell Biology 105:235–246. doi: 10.1083/jcb.105.1.235]
Additional studies revealed that if a sufficiently long spacer sequence separates the N-terminal matrix-targeting sequence and the DHFR portion of the chimeric protein, then, in the presence of methotrexate, a translocation intermediate that spans both membranes can be trapped if enough of the polypeptide protrudes into the matrix to prevent the polypeptide chain from sliding back into the cytosol, possibly by stably associating with matrix Hsp70 (Figure 13-25b). In order for such a stable translocation intermediate to form, the spacer sequence must be long enough to span both membranes; a spacer of 50 amino acids extended to its maximum possible length is adequate to do so. If the chimera contains a shorter spacer—say, 35 amino acids—no stable translocation intermediate is obtained because the spacer cannot span both membranes. These observations provide further evidence that translocated proteins can span both inner and outer mitochondrial membranes and can traverse these membranes only in an unfolded state.
Microscopy studies of stable translocation intermediates show that they accumulate at sites where the inner and outer mitochondrial membranes are close together (Figure 13-25c). Since roughly a thousand stuck chimeric proteins can be observed at these contact sites in a typical yeast mitochondrion, it is thought that mitochondria have approximately a thousand general import pores for the uptake of mitochondrial proteins.
