Pulse-Chase Experiments with Purified ER Membranes Demonstrated That Secreted Proteins Cross the ER Membrane
Although all cells secrete a variety of proteins (e.g., extracellular matrix proteins), certain types of cells are specialized for secretion of large amounts of specific proteins. Pancreatic acinar cells, for instance, synthesize large quantities of several digestive enzymes, which are secreted into ductules that lead to the intestine. Because such secretory cells contain the organelles of the secretory pathway (e.g., ER and Golgi complex) in great abundance, they have been widely used in studying this pathway, including the initial steps that occur at the ER membrane.
The sequence of events that occurs immediately after the synthesis of a secretory protein was first elucidated by pulse-chase experiments with pancreatic acinar cells. In these experiments, radioactively labeled amino acids were incorporated into secretory proteins as they were synthesized on ribosomes bound to the surface of the ER. The portion of the ER that receives proteins entering the secretory pathway is known as the rough ER because it is so densely studded with ribosomes that its surface appears morphologically distinct from other ER membranes (Figure 13-2). From these experiments, it became clear that during or immediately after their synthesis on the ribosome, secretory proteins translocate across the ER membrane into the lumen of the ER.
To delineate the steps in the translocation process, it was necessary to isolate the ER from the rest of the cell. Isolation of intact ER, with its delicate lacelike structure and its interconnectedness with other organelles, is not feasible. However, scientists discovered that when cells are homogenized, the rough ER breaks up into small closed vesicles with ribosomes on the outside, termed microsomes, which retain most of the biochemical properties of the ER, including the capability of protein translocation. The experiments depicted in Figure 13-3, in which microsomes isolated from pulse-labeled cells were treated with a protease, demonstrate that although secretory proteins are synthesized on ribosomes bound to the cytosolic face of the ER membrane, the polypeptides produced by these ribosomes end up within the lumen of a microsome. Experiments such as these raised the question of how polypeptides are recognized as secretory proteins shortly after their synthesis begins and how a nascent secretory protein is threaded across the ER membrane.
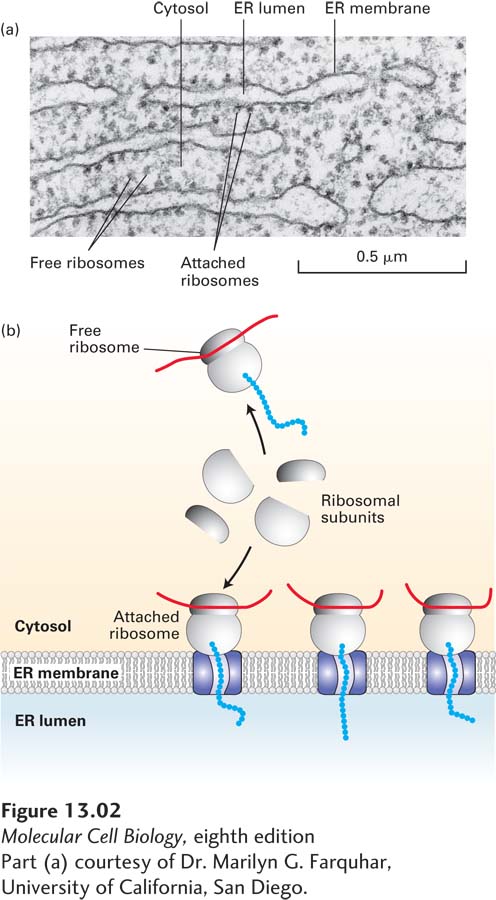
FIGURE 13-2 Structure of the rough ER. (a) Electron micrograph of ribosomes attached to the rough ER in a pancreatic acinar cell. Most of the proteins synthesized by this type of cell are secretory proteins and are formed on membrane-attached ribosomes. A few unattached (free) ribosomes are evident; presumably, these ribosomes are synthesizing cytosolic or other nonsecretory proteins. (b) Schematic representation of protein synthesis on the ER. Note that membrane-bound and free cytosolic ribosomes are identical. Membrane-bound ribosomes are recruited to the ER during synthesis of a polypeptide containing an ER signal sequence.
[Part (a) courtesy of Dr. Marilyn G. Farquhar, University of California, San Diego.]
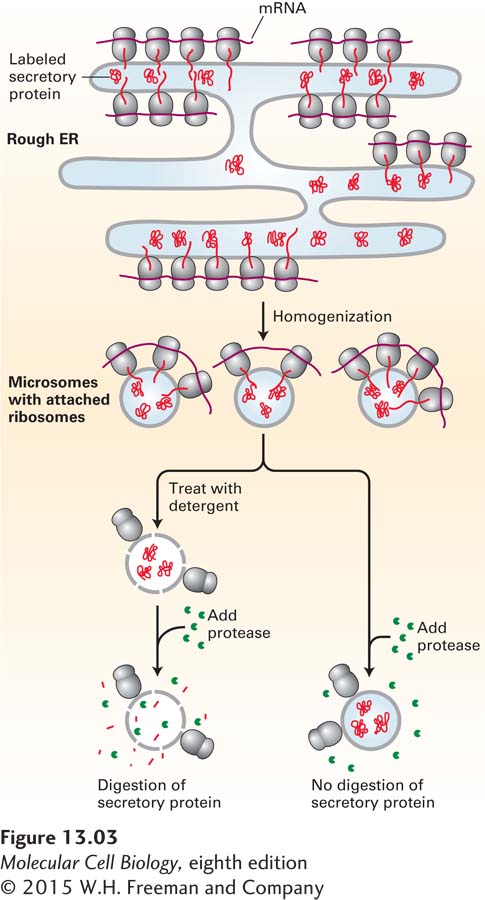
FIGURE 13-3 Secretory proteins enter the ER lumen. Labeling experiments demonstrated that secretory proteins are localized to the ER lumen shortly after synthesis. Cells are incubated for a brief time with radiolabeled amino acids so that only newly synthesized proteins become labeled. The cells are then homogenized, fracturing the plasma membrane and shearing the rough ER into small vesicles called microsomes. Because they have bound ribosomes, microsomes have a much greater buoyant density than other membranous organelles and can be separated from them by a combination of differential and sucrose density-gradient centrifugation (see Chapter 4). The purified microsomes are treated with a protease in the presence or absence of a detergent. The labeled secretory proteins associated with the microsomes are digested by the protease only if the microsomal membrane is first destroyed by treatment with detergent. This finding indicates that the newly made proteins are inside the microsomes, equivalent to the lumen of the rough ER.

