Large and Small Molecules Enter and Leave the Nucleus via Nuclear Pore Complexes
Numerous nuclear pores perforate the nuclear envelope in all eukaryotic cells. Each nuclear pore is formed from an elaborate structure termed the nuclear pore complex (NPC), which is one of the largest protein assemblages in the cell. The total mass of the pore structure is 60,000–80,000 kDa in vertebrates, which is about 16 times larger than a ribosome. An NPC is made up of multiple copies of some 30 different proteins called nucleoporins. Electron micrographs of nuclear pore complexes reveal a membrane-embedded ring structure that surrounds a largely aqueous pore (Figure 13-33). Eight approximately 100-nm-long filaments extend into the nucleoplasm with the distal ends of these filaments joined by a terminal ring, forming a structure called the nuclear basket. Cytoplasmic filaments extend from the cytoplasmic side of the NPC into the cytosol.
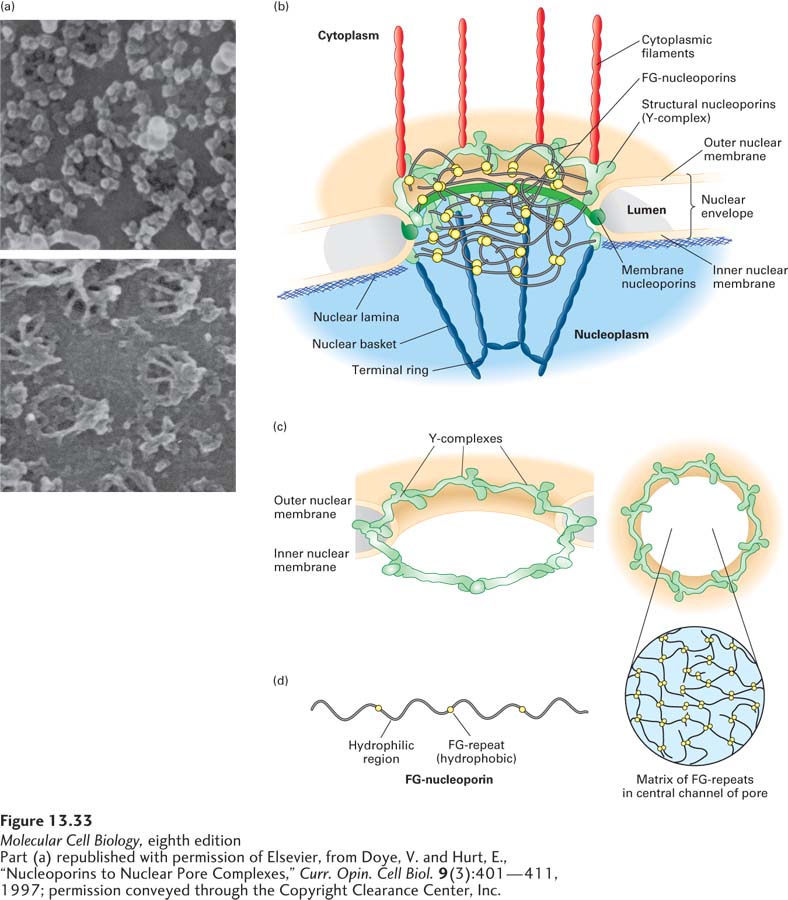
FIGURE 13-33 Nuclear pore complex at different levels of resolution. (a) Nuclear envelopes from the large nuclei of Xenopus oocytes, visualized by scanning electron microscopy. Top: View of the cytoplasmic face reveals the octagonal shape of the membrane-embedded portion of nuclear pore complexes. Bottom: View of the nucleoplasmic face shows the nuclear basket that extends from the membrane-embedded portion. (b) Cutaway model of the nuclear pore complex, showing the major structural features formed by membrane nucleoporins, structural nucleoporins, and FG-nucleoporins. (c) Sixteen copies of the Y-complex form a major part of the structural scaffold of the nuclear pore complex. The three-dimensional structure of the Y-complex is modeled into the pore structure. Note the twofold symmetry across the double membrane of the nucleus (left) and the eightfold rotational symmetry around the axis of the pore (right). (d) The FG-nucleoporins have extended disordered structures that are composed of repeats of the sequence Phe–Gly interspersed with hydrophilic regions (left). The FG-nucleoporins are most abundant in the central part of the pore, and the FG-repeat sequences are thought to fill the central channel with a gel-like matrix (right). See K. Ribbeck and D. Görlich, 2001, EMBO J. 20:1320–1330 and M. P. Rout and J. D. Atchison, 2001, J. Biol. Chem. 276:16593.
[Part (a) republished with permission of Elsevier, from Doye, V. and Hurt, E., “Nucleoporins to Nuclear Pore Complexes,” Curr. Opin. Cell Biol. 9(3):401–411, 1997; permission conveyed through the Copyright Clearance Center, Inc.]
Ions, small metabolites, and globular proteins up to about 40 kDa can diffuse passively through the central aqueous region of the nuclear pore complex. However, large proteins and ribonucleoprotein complexes cannot diffuse in and out of the nucleus. Rather, these macromolecules are actively transported through the NPC with the assistance of soluble transport proteins that bind macromolecules and also interact with nucleoporins. The capacity and efficiency of the NPC for such active transport is remarkable. In one minute, each NPC is estimated to import 60,000 protein molecules into the nucleus, while exporting 50–250 mRNA molecules, 10–20 ribosomal subunits, and 1000 tRNAs out of the nucleus.
In general terms, the nucleoporins are of three types: structural nucleoporins, membrane nucleoporins, and FG-nucleoporins. The structural nucleoporins form the scaffold of the nuclear pore, which is a ring of eightfold rotational symmetry that traverses both membranes of the nuclear envelope, creating an annulus. The inner and outer membranes of the nuclear envelope are connected at the NPC by a highly curved region of membrane that contains the embedded membrane nucleoporins (Figure 13-33b). A set of seven structural nucleoporins forms a Y-shaped structure about the size of the ribosome, known as the Y-complex. Sixteen copies of the Y-complex form the basic structural scaffold of the pore, which has bilateral symmetry across the nuclear envelope and eightfold rotational symmetry in the plane of the envelope (Figure 13-33c). A structural motif repeated several times within the Y-complex is closely related to a structure found in the COPII proteins that drive the formation of coated vesicles within cells (see Chapter 14). This primordial relationship between structural nucleoproteins and vesicle coat proteins suggests that the two types of membrane coat complexes share a common origin. The basic function of this element may be to form a protein lattice that, in a complex with membrane nucleoporins, deforms the membrane into a highly curved structure.
The FG-nucleoporins, which line the channel of the nuclear pore complex and are also found associated with the nuclear basket and the cytoplasmic filaments, contain multiple repeats of short hydrophobic sequences that are rich in phenylalanine (F) and glycine (G) residues (FG-repeats). The hydrophobic FG-repeats are thought to occur in regions of extended, otherwise hydrophilic polypeptide chains that fill the central transporter channel. The FG-nucleoporins are essential for the function of the NPC; however, the NPC remains functional even if up to half of the FG-repeats have been deleted. The FG-nucleoporins are thought to form a flexible gel-like matrix with bulk properties that allow the diffusion of small molecules while excluding unchaperoned hydrophilic proteins larger than 40 kDa (Figure 13-33d).
