Nuclear Transport Receptors Escort Proteins Containing Nuclear-Localization Signals into the Nucleus
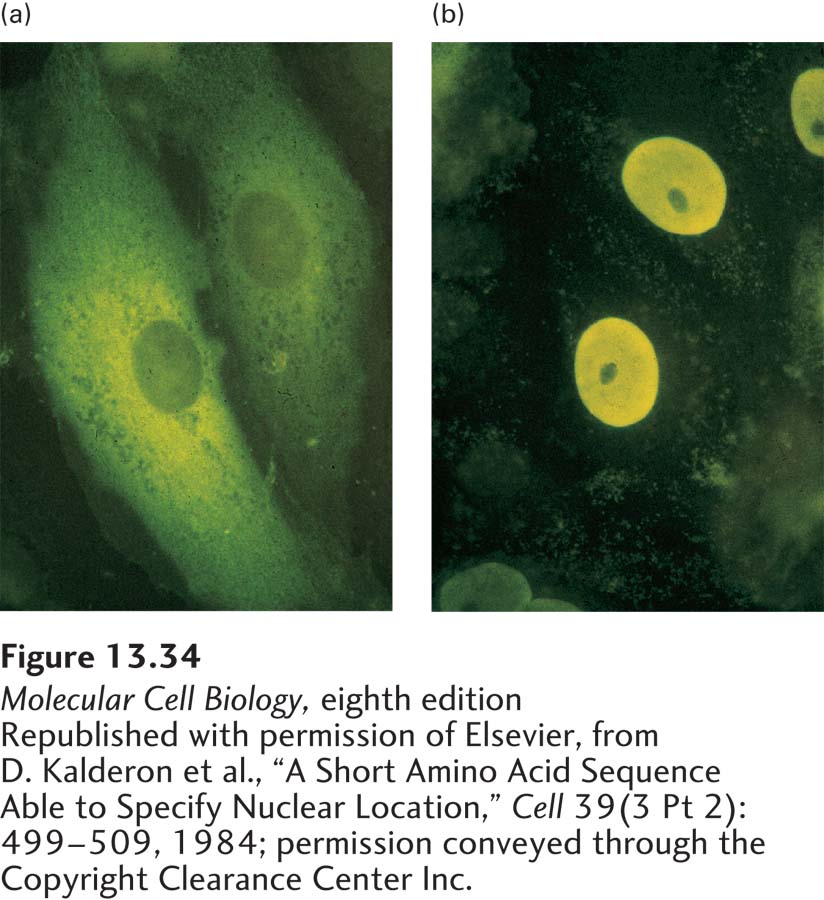
EXPERIMENTAL FIGURE 13-34 Nuclear-localization signals (NLSs) direct proteins to the cell nucleus. Cytoplasmic proteins can be transported to the nucleus if they are fused to a nuclear-localization signal. (a) Normal pyruvate kinase, here visualized by immunofluorescence after cultured cells were treated with a specific antibody (yellow), is localized to the cytoplasm. This very large cytosolic protein functions in carbohydrate metabolism. (b) When a chimeric pyruvate kinase containing the SV40 NLS at its N-terminus was expressed in cells, it was localized to the nucleus. The chimeric protein was expressed from a transfected engineered gene produced by fusing a viral gene fragment encoding the SV40 NLS to the pyruvate kinase gene.
[Republished with permission of Elsevier, from D. Kalderon et al., “A Short Amino Acid Sequence Able to Specify Nuclear Location,” Cell 39(3 Pt 2):499–509, 1984; permission conveyed through the Copyright Clearance Center Inc.]
All proteins found in the nucleus—such as histones, transcription factors, and DNA and RNA polymerases—are synthesized in the cytoplasm and imported into the nucleus through nuclear pore complexes. Such proteins contain a nuclear-localization signal that directs their selective transport into the nucleus. NLSs were first discovered through the analysis of mutations of the gene for large T-antigen encoded by simian virus 40 (SV40). The wild-type form of large T-antigen is localized to the nucleus in virus-infected cells, whereas some mutated forms of large T-antigen accumulate in the cytoplasm (Figure 13-34). The mutations responsible for this altered cellular localization all occur within a specific seven-residue sequence rich in basic amino acids near the C-terminus of the protein: Pro-Lys-Lys-Lys-Arg-Lys-Val. Experiments with chimeric proteins in which this sequence was fused to a cytosolic protein demonstrated that it directs transport into the nucleus and consequently functions as an NLS. NLS sequences were subsequently identified in numerous other proteins imported into the nucleus. Many of these sequences are similar to the basic NLS in SV40 large T-antigen, whereas others are chemically quite different. For instance, an NLS in the RNA-binding protein hnRNP A1 is hydrophobic. Accordingly, there are multiple mechanisms for the recognition of these very different sequences.
Early work on the mechanism of nuclear import showed that proteins containing a basic NLS, similar to the one in SV40 large T-antigen, were efficiently transported into isolated nuclei only if they were provided with a cytosolic extract (Figure 13-35). Using this assay system, researchers purified two required cytosolic components: Ran and a nuclear transport receptor. Ran is a small monomeric G protein that exists in either a GTP-bound or a GDP-bound conformation (see Figure 3-34). It is the cycling of Ran between GTP-bound and GDP-bound conformations, leading to the net hydrolysis of GTP to GDP, that ultimately provides the energy to drive unidirectional transport of macromolecules through the nuclear pore. A nuclear transport receptor binds to the NLS on a cargo protein to be transported into the nucleus and to FG-repeats on nucleoporins. By a physical process that is not well understood, by binding transiently to FG-repeats, nuclear transport receptors have the ability to rapidly traverse the FG-repeat-containing matrix in the central channel of the nuclear pore, whereas proteins of similar size that lack this property are excluded from the central channel. Nuclear transport receptors can be monomeric, consisting of a single polypeptide that can bind both to an NLS and to FG-repeats, or they can be dimeric, with one subunit binding to the NLS and the other binding to FG-repeats.
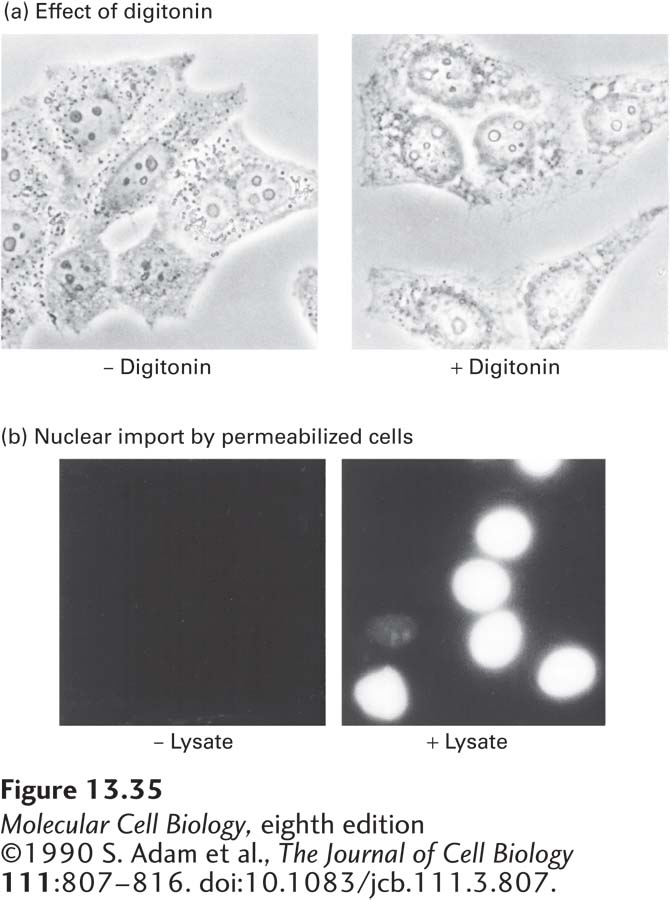
EXPERIMENTAL FIGURE 13-35 Cytosolic proteins are required for nuclear transport. The failure of nuclear transport to occur in permeabilized cultured cells in the absence of cell lysate demonstrates the involvement of soluble cytosolic components in the process. (a) Phase-contrast micrographs of untreated and digitonin-permeabilized HeLa cells. Treatment of a monolayer of cultured cells with the mild non-ionic detergent digitonin permeabilizes the plasma membrane so that cytosolic constituents leak out, but leaves the nuclear envelope and NPCs intact. (b) Fluorescence micrographs of digitonin-permeabilized HeLa cells incubated with a fluorescent protein chemically coupled to a synthetic SV40 T-antigen NLS peptide in the presence and absence of cell lysate (cytosol). Accumulation of the transport substrate in the nucleus occurred only when cytosol was present (right).
[©1990 S. Adam et al., The Journal of Cell Biology 111:807–816. doi:10.1083/jcb.111.3.807.]
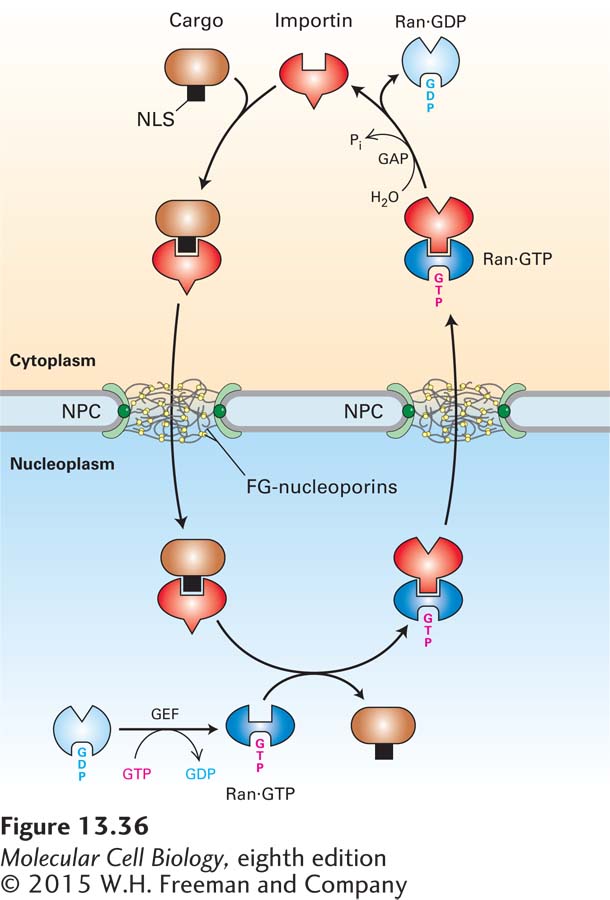
FIGURE 13-36 Mechanism for nuclear import of proteins. In the cytoplasm (top), free importin binds to the NLS of a cargo protein, forming an importin-cargo complex. The importin-cargo complex diffuses through the NPC by transiently interacting with FG-nucleoporins. In the nucleoplasm, Ran·GTP binds to the importin, causing a conformational change that decreases the importin’s affinity for the NLS and releasing the cargo. To support another cycle of import, the importin-Ran·GTP complex is transported back to the cytoplasm. A GTPase-activating protein (GAP) associated with the cytoplasmic filaments of the NPC stimulates Ran to hydrolyze the bound GTP. This generates a conformational change that causes Ran to dissociate from the nuclear transport receptor, which can then initiate another round of import. Ran·GDP is returned to the nucleoplasm, where a guanine nucleotide exchange factor (GEF) causes release of GDP and rebinding of GTP.
The mechanism for the import of cytoplasmic cargo proteins mediated by a nuclear transport receptor known as importin is shown in Figure 13-36. Free importin in the cytoplasm binds to its cognate NLS in a cargo protein, forming an importin-cargo complex. The cargo complex then translocates through the NPC channel as the importin interacts with FG-repeats. The cargo complex rapidly reaches the nucleoplasm, and there the importin interacts with Ran·GTP, which causes a conformational change in the importin that displaces the NLS, releasing the cargo protein into the nucleoplasm. The importin-Ran·GTP complex then diffuses back through the NPC. Once the importin-Ran·GTP complex reaches the cytoplasmic side of the NPC, Ran interacts with a specific GTPase-activating protein (Ran-GAP) that is a component of the NPC cytoplasmic filaments. This interaction stimulates Ran to hydrolyze its bound GTP to GDP, which causes it to convert to a conformation that has low affinity for the importin, so that the importin is released into the cytoplasm, where it can participate in another cycle of import. Ran·GDP travels back through the pore to the nucleoplasm, where it encounters a specific guanine nucleotide exchange factor (Ran-GEF) that causes Ran to release its bound GDP in favor of GTP. The net result of this series of reactions is the coupling of the hydrolysis of GTP to the transfer of an NLS-bearing protein from the cytoplasm to the nuclear interior, thus providing the energy to drive nuclear import.
Although the importin-cargo complex travels through the pore by random diffusion, the overall process of transport of cargo into the nucleus is unidirectional. Because of the rapid dissociation of the complex when it reaches the nucleoplasm, there is a concentration gradient of importin-cargo complex across the NPC: high in the cytoplasm, where the complex assembles, and low in the nucleoplasm, where it dissociates. This concentration gradient is responsible for the unidirectional nature of nuclear import. A similar concentration gradient is responsible for driving importin from the nucleus back into the cytoplasm. The concentration of the importin-Ran·GTP complex is higher in the nucleoplasm, where it assembles, than on the cytoplasmic side of the NPC, where it dissociates. Ultimately, the direction of the transport processes depends on the localization of the Ran-GEF predominantly in the nucleoplasm and the Ran-GAP predominantly in the cytoplasm. Ran-GEF in the nucleoplasm maintains Ran in the Ran·GTP state, where it promotes dissociation of the cargo complex. Ran-GAP on the cytoplasmic side of the NPC converts Ran·GTP to Ran·GDP, dissociating the importin-Ran·GTP complex and releasing free importin into the cytosol.


