A Second Type of Nuclear Transport Receptor Escorts Proteins Containing Nuclear-Export Signals Out of the Nucleus
A mechanism very similar to the one we have just described is used to export proteins, tRNAs, and ribosomal subunits from the nucleus to the cytoplasm. This mechanism was initially elucidated by studies of certain ribonuclear protein complexes that “shuttle” between the nucleus and the cytoplasm. Such “shuttling” proteins contain a nuclear-export signal (NES) that stimulates their export from the nucleus to the cytoplasm through nuclear pores, in addition to an NLS that results in their uptake into the nucleus. Experiments with engineered hybrid genes encoding a nucleus-restricted protein fused to various segments of a shuttling protein have identified at least three different types of NESs: a leucine-rich sequence found in PKI (an inhibitor of protein kinase A) and in the Rev protein of human immunodeficiency virus (HIV), and two other sequences identified in two different heterogeneous ribonucleoprotein particles (hnRNPs). The precise structural features that determine the recognition of each type of sequence for nuclear export remain poorly understood.
The mechanism whereby shuttling proteins are exported from the nucleus is best understood for those containing a leucine-rich NES. According to the current model, shown in Figure 13-37a, a specific nuclear transport receptor, called exportin 1, first forms a complex with Ran·GTP in the nucleus and then binds the NES in a cargo protein. Binding of exportin 1 to Ran·GTP causes a conformational change in exportin 1 that increases its affinity for the NES, so that a trimolecular cargo complex is formed. Like other nuclear transport receptors, exportin 1 interacts transiently with FG-repeats in FG-nucleoporins and diffuses through the NPC. The cargo complex dissociates when it encounters the Ran-GAP associated with the NPC cytoplasmic filaments, which stimulates Ran to hydrolyze the bound GTP, shifting it into a conformation that has low affinity for exportin 1. After Ran·GDP dissociates from the trimolecular cargo complex, exportin 1 changes its conformation to one that has low affinity for the NES, releasing the cargo into the cytosol. The direction of the export process is driven by this dissociation of the cargo from exportin 1 in the cytoplasm, which causes a concentration gradient of the cargo complex across the NPC that is high in the nucleoplasm and low in the cytoplasm. Exportin 1 and Ran·GDP are then transported back into the nucleus through the NPC.
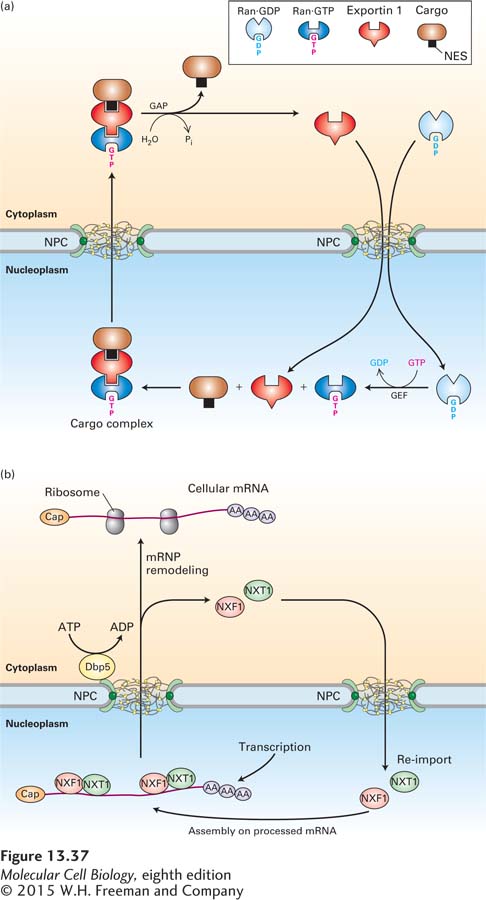
FIGURE 13-37 Ran-dependent and Ran-independent nuclear export. (a) Ran-dependent mechanism for nuclear export of cargo proteins containing a leucine-rich nuclear-export signal (NES). In the nucleoplasm (bottom), the protein exportin 1 binds cooperatively to the NES of the cargo protein to be transported and to Ran·GTP. After the resulting cargo complex diffuses through an NPC via transient interactions with FG-repeats in FG-nucleoporins, the GAP associated with the NPC cytoplasmic filaments stimulates GTP hydrolysis, converting Ran·GTP to Ran·GDP. The accompanying conformational change in Ran leads to dissociation of the complex. The NES-containing cargo protein is released into the cytosol, whereas exportin 1 and Ran·GDP are transported back into the nucleus through an NPC. Ran-GEF in the nucleoplasm then stimulates conversion of Ran·GDP to Ran·GTP. (b) Ran-independent nuclear export of mRNAs. The heterodimeric NXF1/NXT1 complex binds to mRNA-protein complexes (mRNPs) in the nucleus. NXF1/NXT1 acts as a nuclear transport receptor and directs the associated mRNP to the central channel of the NPC by transiently interacting with FG-nucleoporins. An RNA helicase (Dbp5) located on the cytoplasmic side of the NPC removes NXF1 and NXT1 from the mRNA in a reaction that is powered by ATP hydrolysis. Free NXF1 and NXT1 proteins are recycled back into the nucleus by the Ran-dependent import process depicted in Figure 13-36.
By comparing this model for nuclear export with that in Figure 13-36 for nuclear import, we can see one obvious difference: Ran·GTP is part of the cargo complex during export, but not during import. Apart from this difference, the two transport processes are remarkably similar. In both processes, association of a nuclear transport receptor with Ran·GTP in the nucleoplasm causes a conformational change that affects its affinity for the transport signal. During import, the interaction causes release of the cargo, whereas during export, the interaction promotes association with the cargo. In both export and import, stimulation of Ran·GTP hydrolysis in the cytoplasm by Ran-GAP produces a conformational change in Ran that releases the nuclear transport receptor. During nuclear export, the cargo is also released. Localization of Ran-GAP and Ran-GEF to the cytoplasm and nucleus, respectively, is the basis for the unidirectional import and export of cargo proteins across the NPC.
In keeping with their similarity in function, the two types of nuclear transport receptors—importins and exportins—are highly homologous in sequence and structure. The family of nuclear transport receptors has 14 members in yeast and more than 20 in mammalian cells. The NESs or NLSs to which they bind have been determined for only a fraction of them. Some individual nuclear transport receptors function in both import and export.
A similar shuttling mechanism has been shown to export other cargoes from the nucleus. For example, exportin-t functions to export tRNAs. Exportin-t binds fully processed tRNAs in a complex with Ran·GTP that diffuses through NPCs and dissociates when it interacts with Ran-GAP in the NPC cytoplasmic filaments, releasing the tRNA into the cytosol. A Ran-dependent process is also required for the nuclear export of ribosomal subunits through NPCs once the protein and RNA components have been properly assembled in the nucleolus. Likewise, certain specific mRNAs that associate with particular hnRNP proteins can be exported by a Ran-dependent mechanism.
