Mannose 6-Phosphate Residues Target Soluble Proteins to Lysosomes
As we have seen, many of the sorting signals that direct cargo-protein trafficking in the secretory pathway are short amino acid sequences in the targeted protein. In contrast, the sorting signal that directs soluble lysosomal enzymes from the trans-Golgi network to the late endosome is a carbohydrate residue, mannose 6-phosphate (M6P), which is formed in the cis-Golgi. The addition and initial processing of one or more preformed N-linked oligosaccharide precursors in the rough ER is the same for lysosomal enzymes as for membrane and secreted proteins, yielding core Man8(GlcNAc)2 chains (see Figure 13-18). In the cis-Golgi, the N-linked oligosaccharides present on most lysosomal enzymes undergo a two-step reaction sequence that generates M6P residues (Figure 14-21). The addition of M6P residues to the oligosaccharide chains of soluble lysosomal enzymes prevents these proteins from undergoing the further processing reactions characteristic of secreted and membrane proteins (see Figure 14-14).
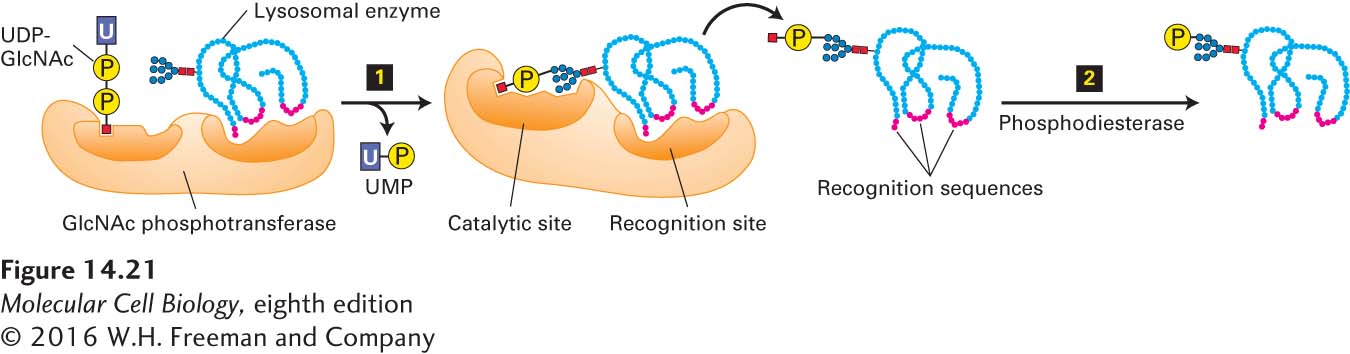
FIGURE 14-21 Formation of mannose 6-phosphate (M6P) residues that target soluble enzymes to lysosomes. The M6P residues that direct proteins to lysosomes are generated in the cis-Golgi by two Golgi-resident enzymes. Step 1: An N-acetylglucosamine (GlcNAc) phosphotransferase transfers a phosphorylated GlcNAc group to carbon atom 6 of one or more mannose residues. Because only lysosomal enzymes contain sequences (red) that are recognized and bound by this enzyme, phosphorylated GlcNAc groups are added specifically to lysosomal enzymes. Step 2: After release of the modified protein from the phosphotransferase, a phosphodiesterase removes the GlcNAc group, leaving a phosphorylated mannose residue on the lysosomal enzyme. See A. B. Cantor et al., 1992, J. Biol. Chem. 267:23349, and S. Kornfeld, 1987, FASEB J. 1:462.
As shown in Figure 14-22, the segregation of M6P-bearing lysosomal enzymes from secreted and membrane proteins occurs in the trans-Golgi network. Here transmembrane mannose 6-phosphate receptors bind the M6P residues on lysosome-destined proteins very tightly and specifically. Clathrin/AP1-coated vesicles containing the M6P receptor and bound lysosomal enzymes then bud from the trans-Golgi network, lose their coats, and subsequently fuse with a late endosome by mechanisms described previously. Because M6P receptors can bind M6P at the slightly acidic pH (∼6.5) of the trans-Golgi network, but not at a pH of less than 6, the bound lysosomal enzymes are released within late endosomes, which have an internal pH of 5.0–5.5. Furthermore, a phosphatase within late endosomes usually removes the phosphate from M6P residues on lysosomal enzymes, preventing any rebinding to the M6P receptor that might occur in spite of the low pH there. Vesicles budding from late endosomes, known as retromers, recycle the M6P receptor back to the trans-Golgi network. Eventually, mature late endosomes fuse with lysosomes, delivering the lysosomal enzymes to their final destination.
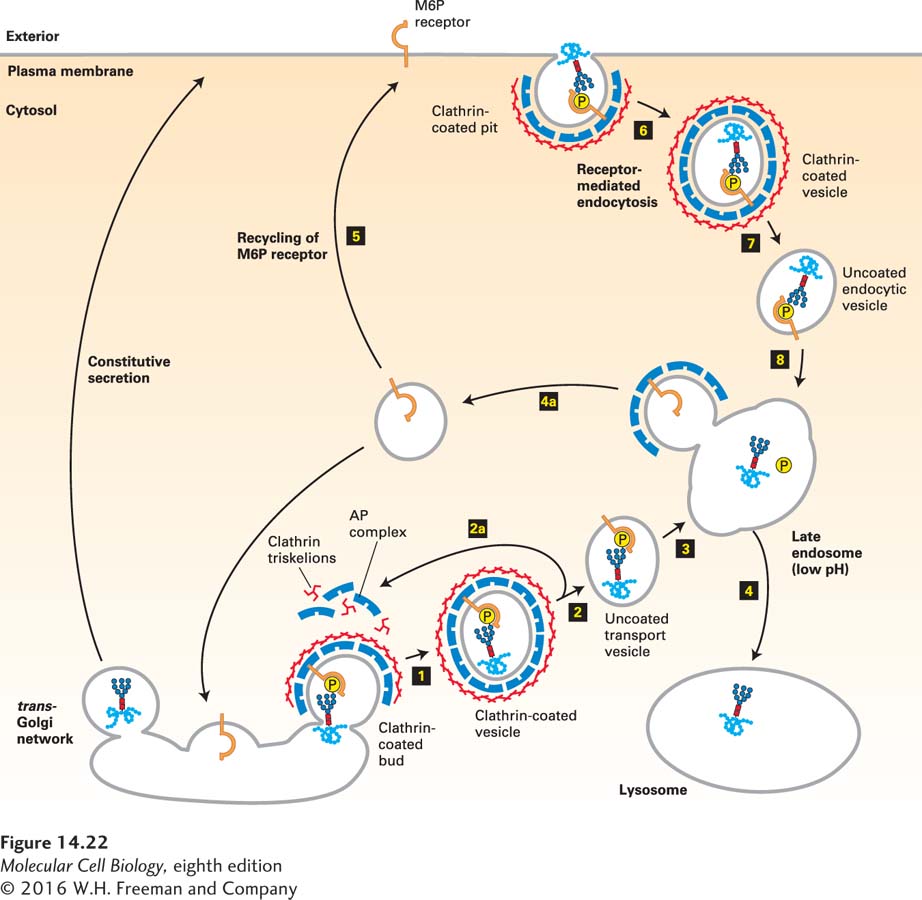
FIGURE 14-22 Trafficking of soluble lysosomal enzymes from the trans-Golgi network and cell surface to lysosomes. Newly synthesized lysosomal enzymes, produced in the ER, acquire mannose 6-phosphate (M6P) residues in the cis-Golgi (see Figure 14-21). For simplicity, only one phosphorylated oligosaccharide chain is depicted, although lysosomal enzymes typically have many such chains. In the trans-Golgi network, proteins that bear the M6P sorting signal interact with M6P receptors in the membrane and thereby are directed into clathrin/AP1-coated vesicles (step 1). The coat surrounding released vesicles is rapidly depolymerized (step 2), and the uncoated transport vesicles fuse with late endosomes (step 3). After the phosphorylated enzymes dissociate from the M6P receptors and are dephosphorylated, late endosomes subsequently fuse with a lysosome (step 4). Note that coat proteins and M6P receptors are recycled (steps 2a and 4a), and that some receptors are delivered to the cell surface (step 5). Phosphorylated lysosomal enzymes are occasionally sorted from the trans-Golgi to the cell surface and secreted. These secreted enzymes can be retrieved by receptor-mediated endocytosis (steps 6–8), a process that closely parallels trafficking of lysosomal enzymes from the trans-Golgi network to lysosomes. See G. Griffiths et al., 1988, Cell 52:329; S. Kornfeld, 1992, Annu. Rev. Biochem. 61:307; and G. Griffiths and J. Gruenberg, 1991, Trends Cell Biol. 1:5.
The sorting of soluble lysosomal enzymes in the trans-Golgi network (Figure 14-22, steps 1–4) shares many features with the trafficking of proteins between the ER and cis-Golgi compartments mediated by COPII and COPI vesicles. First, M6P acts as a sorting signal by interacting with the luminal domain of a receptor protein in the donor membrane. Second, the membrane-embedded receptors with their bound ligands are incorporated into the appropriate vesicles—in this case, either GGA- or AP1-containing clathrin-coated vesicles—by interacting with the vesicle coat. Third, these transport vesicles fuse with only one specific organelle, here the late endosome, as the result of interactions between specific v-SNAREs and t-SNAREs. And finally, intracellular transport receptors dissociated from their bound ligand are recycled by retrograde vesicle trafficking.

