Receptors for Macromolecular Ligands Contain Sorting Signals That Target Them for Endocytosis
The key to understanding how LDL particles bind to the cell surface and are then taken up into endocytic vesicles was the discovery of the LDL receptor. The LDL receptor is an 839-residue glycoprotein with a single transmembrane segment; it has a short C-terminal cytosolic segment and a long N-terminal exoplasmic segment that contains the LDL-binding domain. Seven cysteine-rich repeats form the LDL-binding domain, which interacts with the apoB-100 molecule in an LDL particle. Figure 14-29 shows how the LDL receptor facilitates internalization of LDL particles by receptor-mediated endocytosis. After internalized LDL particles reach lysosomes, lysosomal proteases hydrolyze their surface apolipoproteins and lysosomal cholesteryl esterases hydrolyze their core cholesteryl esters. The unesterified cholesterol is then free to leave the lysosome and be used as necessary by the cell in the synthesis of membranes or various cholesterol derivatives.
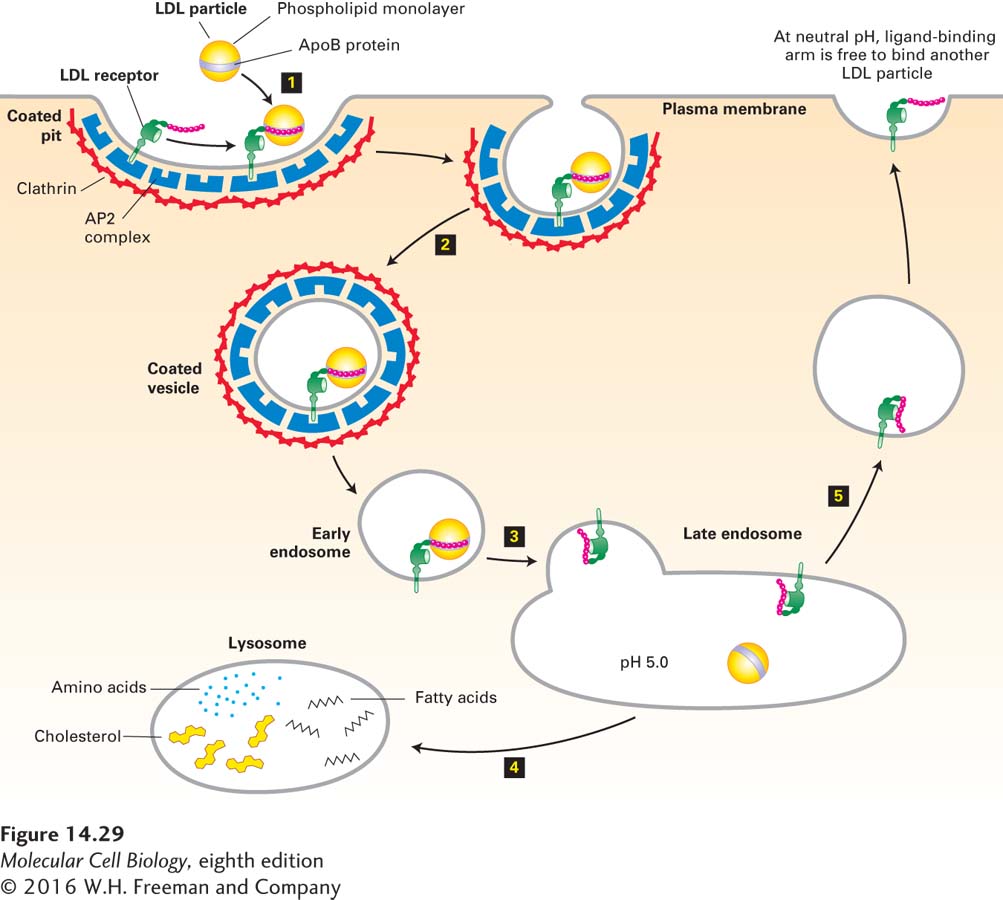
FIGURE 14-29 Endocytic pathway for internalizing low-density lipoprotein (LDL). Step 1: A cell-surface LDL receptor binds to an ApoB protein embedded in the phospholipid outer layer of an LDL particle. Interaction between the NPXY sorting signal in the cytosolic tail of the LDL receptor and the AP2 complex incorporates the receptor-ligand complex into a forming endocytic vesicle. Step 2: Clathrin-coated pits containing receptor-LDL complexes are pinched off by the same dynamin-mediated mechanism used to form clathrin/AP1-coated vesicles on the trans-Golgi network (see Figure 14-19). Step 3: After the vesicle coat is shed, the uncoated endocytic vesicle (early endosome) fuses with a late endosome. The acidic pH in this compartment causes a conformational change in the LDL receptor that leads to release of the bound LDL particle. Step 4: The late endosome fuses with a lysosome, and the proteins and lipids of the free LDL particle are broken down into their constituent parts by enzymes in the lysosome. Step 5: The LDL receptor is recycled to the cell surface, where at the neutral pH of the exterior medium, the receptor undergoes a conformational change so that it can bind another LDL particle. See M. S. Brown and J. L. Goldstein, 1986, Science 232:34, and G. Rudenko et al., 2002, Science 298:2353.
The discovery of the LDL receptor and an understanding of how it functions came from studying cells from patients with familial hypercholesterolemia (FH), a hereditary disease that is marked by elevated plasma LDL levels and is now known to be caused by mutations in the LDL receptor (LDLR) gene. In patients who have one normal and one defective copy of the LDLR gene (heterozygotes), LDL in the blood is increased about twofold. Those with two defective LDLR genes (homozygotes) have LDL levels that are from fourfold to sixfold higher than normal. Without medical intervention, FH heterozygotes commonly develop cardiovascular disease about 10 years earlier than normal people do, and FH homozygotes usually die of heart attacks before reaching their late twenties.
A variety of mutations in the LDLR gene can cause FH. Some mutations prevent the synthesis of the LDLR protein; others prevent proper folding of the receptor protein in the ER, leading to its premature degradation (see Chapter 13); and still other mutations reduce the ability of the LDL receptor to bind LDL tightly. A particularly informative group of mutant receptors are expressed on the cell surface and bind LDL normally but cannot mediate the internalization of bound LDL. In individuals with this type of defect, plasma-membrane receptors for other ligands are internalized normally, but the mutant LDL receptor is not recruited into coated pits. Analysis of this mutant receptor and other mutant LDL receptors generated experimentally and expressed in fibroblasts identified a four-residue motif in the cytosolic segment of the receptor that is crucial for its internalization: Asn-Pro-X-Tyr, where X can be any amino acid. This NPXY sorting signal binds to the AP2 complex, linking the clathrin/AP2 coat to the cytosolic segment of the LDL receptor in coated pits. A mutation in any of the conserved residues of the NPXY signal abolishes the ability of the LDL receptor to be incorporated into coated pits.
A small number of individuals who exhibit the usual symptoms associated with FH produce normal LDL receptors. In these individuals, the gene encoding the AP2 subunit protein that binds the NPXY sorting signal is defective. As a result, LDL receptors are not incorporated into clathrin/AP2-coated vesicles, and endocytosis of LDL particles is compromised. Analyses of patients with this genetic disorder highlight the importance of adapter proteins in protein trafficking mediated by clathrin-coated vesicles.
Mutational studies have shown that other cell-surface receptors can be directed into budding clathrin/AP2-coated pits by a YXXΦ sorting signal. Recall from our earlier discussion that this same sorting signal recruits membrane proteins into clathrin/AP1-coated vesicles that bud from the trans-Golgi network by binding to a subunit of AP1 (see Table 14-2). All these observations indicate that YXXΦ is a widely used signal for sorting membrane proteins to clathrin-coated vesicles.
In some cell-surface proteins, however, other sequences (e.g., Leu-Leu) or covalently linked ubiquitin molecules act as signals for endocytosis. Among the proteins associated with clathrin/AP2-coated vesicles, several contain domains that bind specifically to ubiquitin, and it has been hypothesized that these vesicle-associated proteins mediate the selective incorporation of ubiquitinylated membrane proteins into endocytic vesicles. As described later, the ubiquitin tag on endocytosed membrane proteins is also recognized at a later stage in the endocytic pathway and plays a role in delivering these proteins into the interior of the lysosome, where they are degraded.
