The Autophagic Pathway Delivers Cytosolic Proteins or Entire Organelles to Lysosomes
When cells are placed under stressful conditions, such as starvation, they have the capacity to recycle macromolecules for use as nutrients in a process of lysosomal degradation known as autophagy (“eating oneself”). The autophagic pathway begins with the formation of a flattened double-membrane cup-shaped structure that envelops a region of the cytosol or an entire organelle (e.g., a mitochondrion), forming an autophagosome, or autophagic vesicle (Figure 14-35). The outer membrane of an autophagosome can fuse with a lysosome, delivering a large vesicle, bounded by a single membrane bilayer, to the interior of the lysosome. Lipases and proteases within the lysosome degrade the autophagosome and its contents into their molecular components, just as they do when the contents of multivesicular endosomes are delivered to the lysosome. Amino acid permeases in the lysosomal membrane then allow for the transport of free amino acids back into the cytosol for use in synthesis of new proteins.
By studying mutants with defects in the autophagic pathway, scientists have identified processes other than recycling of cellular components during starvation that also depend on autophagy. Experiments carried out principally in Drosophila and mice have shown that autophagy participates in a type of quality-control mechanism that removes organelles that have ceased to function properly. In particular, the autophagic pathway can target dysfunctional mitochondria that have lost their integrity and no longer have an electrochemical gradient across their inner membrane. In certain cell types, pathogenic bacteria and viruses that are multiplying in the cytosol of host cells can be targeted to the autophagic pathway for destruction in the lysosome as part of a host defense mechanism against infection.
In each of these processes, and in all eukaryotic organisms, the autophagic pathway takes place in three basic steps. Although the mechanisms underlying each of these steps are relatively poorly understood, they are thought to be related to the basic mechanisms for vesicular trafficking discussed in this chapter.
Autophagosome Nucleation The autophagosome is thought to originate from a fragment of a membrane-bounded organelle. The origin of this membrane has been difficult to trace because no known integral membrane proteins, which might serve to identify the source of this membrane, are known to be required for the formation of the autophagosome. Studies in yeast have shown that some mutants defective in Golgi trafficking are also defective in autophagy, suggesting that the autophagosome is initially derived from a fragment of the Golgi. Autophagy that is induced by starvation appears to be a nonspecific process in which a random portion of the cytoplasm, including organelles, becomes enveloped by an autophagosome. In these cases, the site of nucleation is probably random. In cases in which defective organelles are enveloped by the autophagosome, some type of signal or binding site must be present on the surface of the organelle to target nucleation of the autophagosome.
Autophagosome Growth and Completion New membrane must be delivered to the autophagosome membrane in order for this cup-shaped organelle to grow. This growth probably occurs by the fusion of transport vesicles with the membrane of the autophagosome. About 30 proteins that participate in the formation of autophagosomes have been identified in genetic screens for yeast mutants that are defective in autophagy. One of these proteins is Atg8, shown in Figure 14-35, which is covalently linked to the lipid phosphatidylethanolamine and thus becomes attached to the cytoplasmic face of the autophagosome. Association of Atg8 with a membrane vesicle appears to be the key step in enabling a vesicle to fuse with the growing autophagosome.
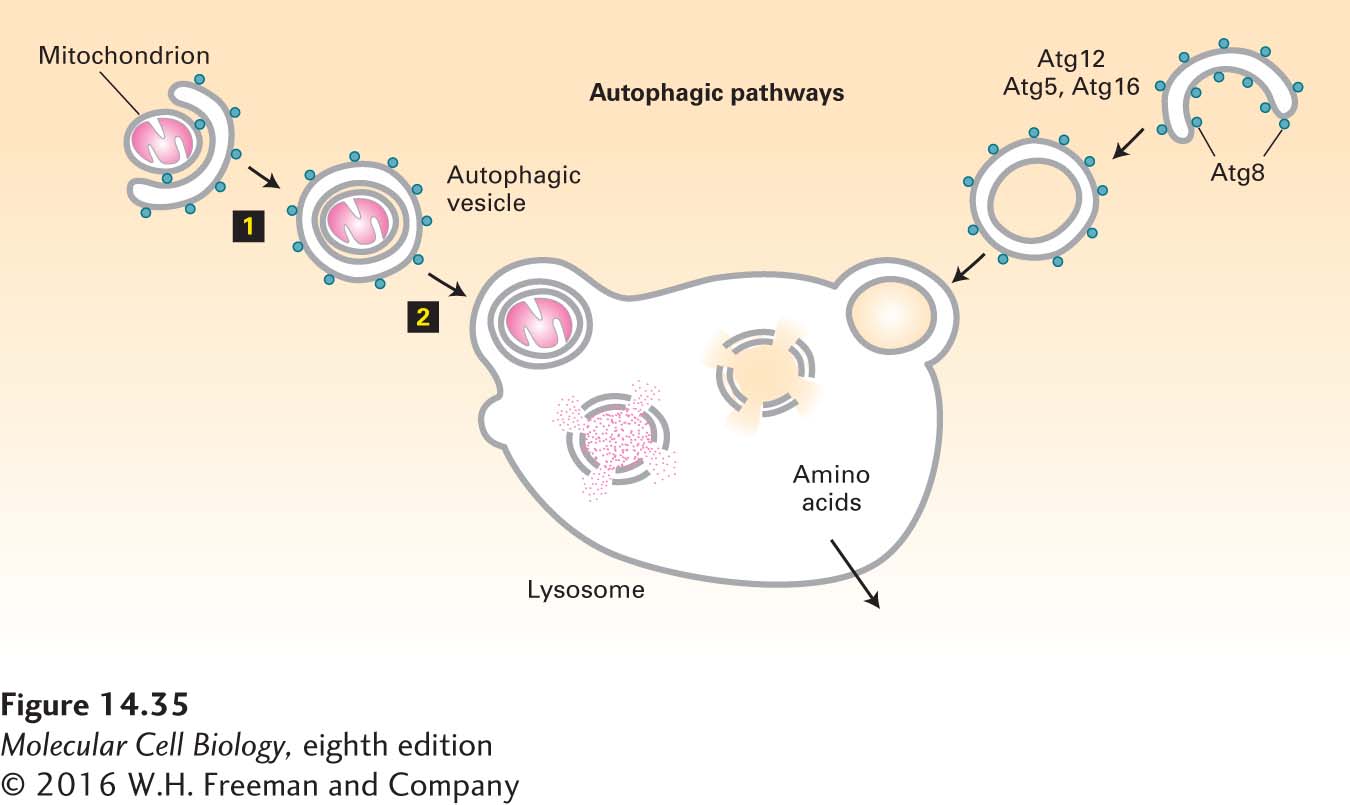
FIGURE 14-35 The autophagic pathway. The autophagic pathway allows cytosolic proteins and organelles to be delivered to the lysosome interior for degradation. In the autophagic pathway, a cup-shaped structure forms around a portion of the cytosol (right) or an organelle such as a mitochondrion (left). Continued addition of membrane eventually leads to the formation of an autophagosome that envelops its contents in two complete membranes (step 1). Fusion of the outer membrane with the membrane of a lysosome releases a single-membrane vesicle and its contents into the lysosome interior (step 2). After degradation of the protein and lipid components by hydrolases in the lysosome interior, the released amino acids are transported across the lysosomal membrane into the cytosol. Proteins known to participate in the autophagic pathway include Atg8, which forms a coat structure around the autophagosome.
Fusion of Atg8-containing vesicles with the autophagosome involves the formation of a cytosolic assembly of Atg12, Atg5, and Atg16. Atg12 is similar in structure to ubiquitin, and a set of proteins related to ubiquitin-conjugating enzymes are responsible for covalently joining Atg12 to Atg5 by a process similar to that used for covalently joining ubiquitin to a target protein (see Figure 3-31). The covalently linked Atg12-Atg5 dimer then co-assembles with Atg16 to form a polymeric complex localized to the site of a growing autophagosome. By an unknown mechanism, this cytosolic complex is thought to bring about the fusion of Atg8-containing vesicles into a cup-shaped autophagosome.
Autophagosome Targeting and Fusion The outer membrane of the completed autophagosome is thought to contain a set of proteins that target it for fusion with the membrane of a lysosome. Two vesicle-tethering proteins have been found to be required for autophagosome fusion with a lysosome, but the corresponding SNARE proteins have not been identified. Fusion of the autophagosome with the lysosome occurs after Atg8 has been released from the autophagosome membrane by proteolytic cleavage, and this proteolysis step occurs only after the autophagosome has completely formed a sealed double-membrane system. Thus Atg8 protein appears to mask fusion proteins and to prevent premature fusion of the autophagosome with the lysosome.
