Yeast Mutants Define Major Stages and Many Components in Vesicular Transport
The general organization of the secretory pathway and many of the molecular components required for vesicle trafficking are similar in all eukaryotic cells. Because of this conservation, genetic studies with yeast have been useful in confirming the sequence of steps in the secretory pathway and in identifying many of the proteins that participate in vesicular traffic. For yeast cells, as for all cells, the secretory pathway is essential for transport and delivery of new protein and membrane to the cell surface. Thus genes encoding important components of the secretory pathway are essential for cell growth and can be studied only as conditional mutants, as described in Chapter 8. Although yeasts secrete few proteins into the growth medium, they continuously secrete a number of enzymes that remain localized in the narrow space between the plasma membrane and the cell wall. The best studied of these, invertase, hydrolyzes the disaccharide sucrose to glucose and fructose.
636
A large number of yeast mutants were initially identified by their ability to secrete proteins at one temperature and their inability to do so at a higher, nonpermissive temperature. When these temperature-
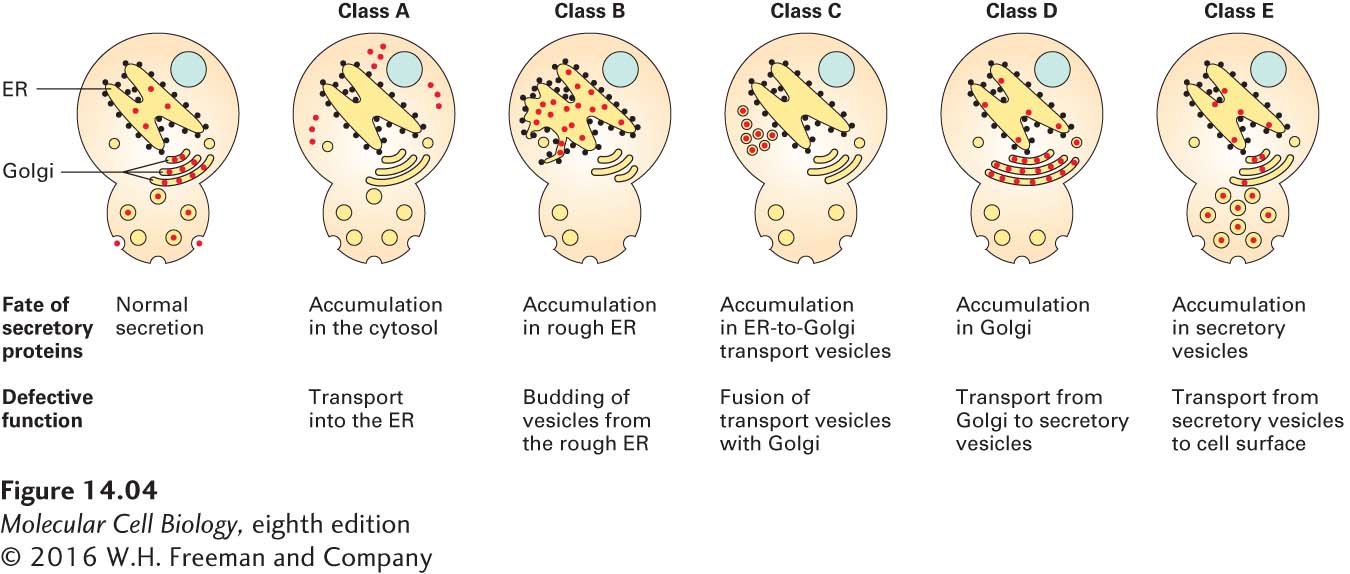
To determine the order of the steps in the pathway, researchers analyzed double sec mutants. For instance, when yeast cells contain mutations in both class B and class D functions, proteins accumulate in the rough ER, not in the Golgi cisternae. Because proteins accumulate at the earliest blocked step, this finding shows that class B mutations must act at an earlier point in the secretory pathway than class D mutations do. These studies confirmed that as a secreted protein is synthesized and processed, it moves sequentially from the cytosol to the rough ER, to ER-
The three methods outlined in this section have delineated the major steps of the secretory pathway and have contributed to the identification of many of the proteins responsible for vesicle budding and fusion. Each of the individual steps in the secretory pathway is currently being studied in mechanistic detail, and increasingly, biochemical assays and molecular genetic studies are being used to study each of these steps in terms of the function of individual protein molecules.