Classic Experiment 14-1: Following a Protein Out of the Cell
Following a Protein Out of the Cell
J. Jamieson and G. Palade, 1966, P. Natl. Acad. Sci. USA 55(2):424–
The advent of electron microscopy allowed researchers to see the cell and its structures at an unprecedented level of detail. George Palade used this tool not only to look at the fine details of the cell, but also to analyze the process of secretion. By combining electron microscopy with pulse-
Background
In addition to synthesizing proteins to carry out cellular functions, many cells must also produce and secrete additional proteins that perform their duties outside the cell. Cell biologists, including Palade, wondered how secreted proteins make their passage from the inside to the outside of the cell. Early experiments whose results suggested that proteins destined for secretion are synthesized in a particular intracellular location and then follow a pathway to the cell surface employed methods to disrupt cells synthesizing a particular secreted protein and to separate their various organelles by centrifugation. These cell-
The Experiment
Palade wanted to identify the cell structures and organelles that participate in protein secretion. To study this complex process, he carefully chose an appropriate model system, the pancreatic acinar cell, which is responsible for producing and secreting large amounts of digestive enzymes. Because these cells have the unusual property of expressing only secretory proteins, a general label for newly synthesized protein, such as radioactively labeled leucine, would only be incorporated into protein molecules that were following the secretory pathway.
Palade first examined the protein secretory pathway in vivo by injecting live guinea pigs with [3H]leucine, which was incorporated into newly made proteins, thereby radioactively labeling them. At time points from 4 minutes to 15 hours after injection, the animals were sacrificed and the pancreatic tissue was fixed. By subjecting the specimens to autoradiography and viewing them in an electron microscope, Palade could trace where in cells the labeled proteins were located at various times. As expected, the radioactivity was localized in vesicles at the ER immediately following the [3H]leucine injection and at the plasma membrane at the later time points. The surprise came in the middle time points. Rather than traveling straight from the ER to the plasma membrane, the radioactively labeled proteins appeared to stop off at the Golgi complex in the middle of their journey. In addition, there was no time point at which the radioactively labeled proteins were not confined to vesicles.
The observation that the Golgi complex was involved in protein secretion was both surprising and intriguing. To thoroughly address the role of this organelle in protein secretion, Palade turned to in vitro pulse-
Once convinced that his in vitro system accurately mimicked protein secretion in vivo, Palade proceeded to the critical experiment. He pulse-
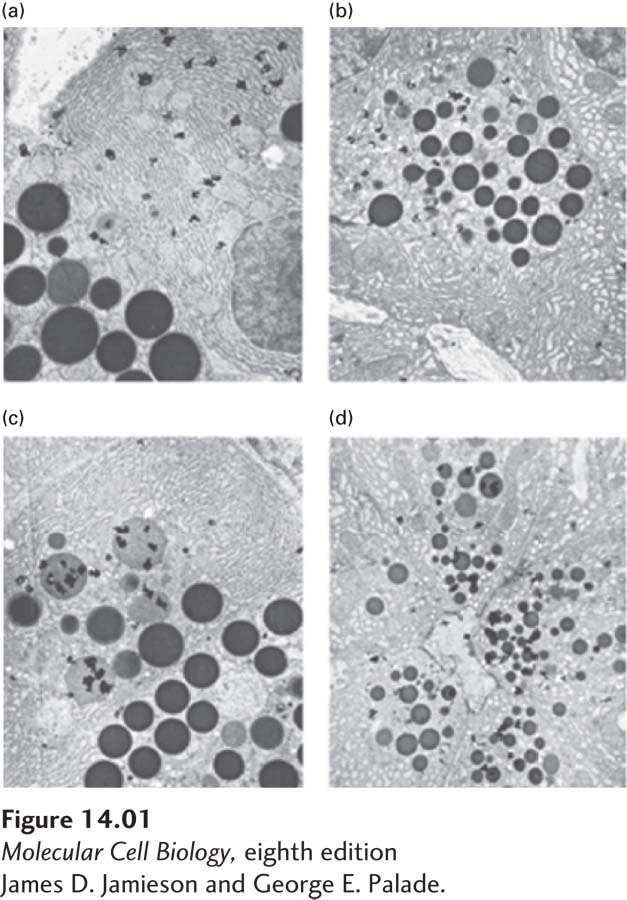
Discussion
Palade’s experiments gave biologists the first clear look at the stages of the secretory pathway. His studies on pancreatic acinar cells yielded two fundamental observations. First, he showed that secreted proteins pass through the Golgi complex on their way out of the cell. This was the first function assigned to the Golgi complex. Second, he showed that secreted proteins never mix with cellular proteins in the cytosol; they are segregated in vesicles throughout the secretory pathway. These findings were predicated on two important aspects of the experimental design. Palade’s careful use of electron microscopy and autoradiography allowed him to look at the fine details of the pathway. Of equal importance was his choice of a cell type devoted to secretion, the pancreatic acinar cell, as a model system. In a different cell type, significant amounts of nonsecretory proteins would have also been produced during the labeling, obscuring the fate of secretory proteins in particular.
Palade’s work set the stage for more detailed studies. Once the secretory pathway had been clearly described, entire fields of research were opened up to investigation of the synthesis and movement of both secreted and membrane proteins. For this groundbreaking work, Palade was awarded the Nobel Prize in Physiology or Medicine in 1974.