Assembly of a Protein Coat Drives Vesicle Formation and Selection of Cargo Molecules
The budding of a vesicle from its parent membrane is driven by the polymerization of soluble protein complexes on the membrane to form a proteinaceous vesicle coat (Figure 14-6a). Interactions between the cytosolic portions of integral membrane proteins and the vesicle coat gather the appropriate cargo proteins into the forming vesicle. Thus the coat gives curvature to the membrane to form a vesicle and acts as the filter to determine which proteins are admitted into the vesicle.
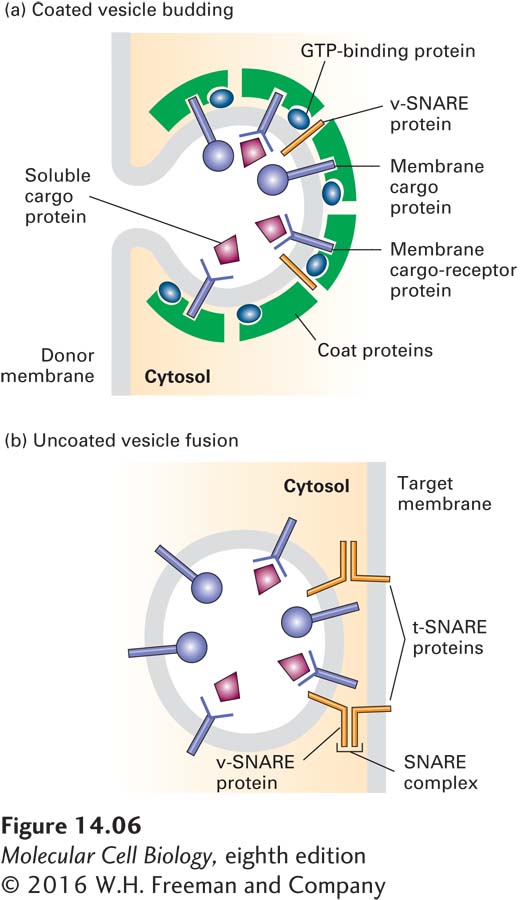
Proteins responsible for the eventual fusion of a vesicle with the target membrane, known as v-
Three major types of coated vesicles have been characterized, each with a different type of protein coat and each formed by reversible polymerization of a distinct set of protein subunits (Table 14-1). Each type of vesicle, named for its primary coat proteins, transports cargo proteins from particular parent organelles to particular target organelles:
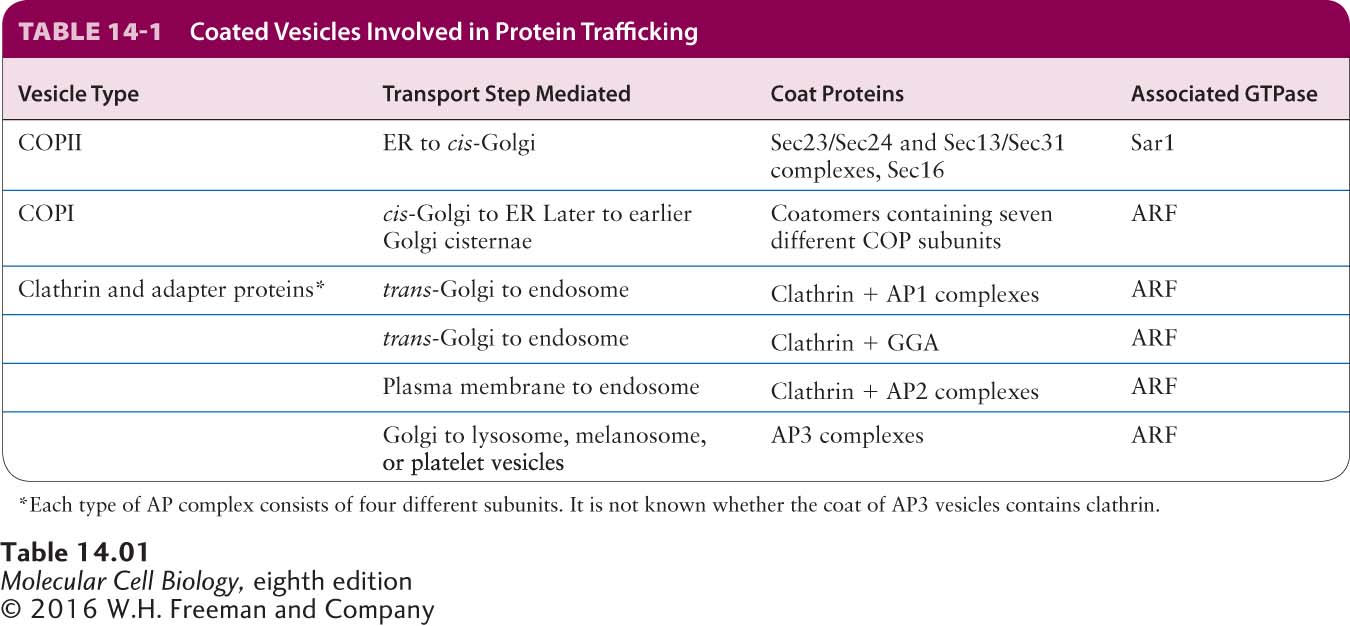
COPII vesicles transport proteins from the ER to the Golgi.
COPI vesicles mainly transport proteins in the retrograde direction between Golgi cisternae and from the cis-Golgi back to the ER.
Clathrin-coated vesicles transport proteins from the plasma membrane (cell surface) and the trans-Golgi network to late endosomes.
Every vesicle-
639
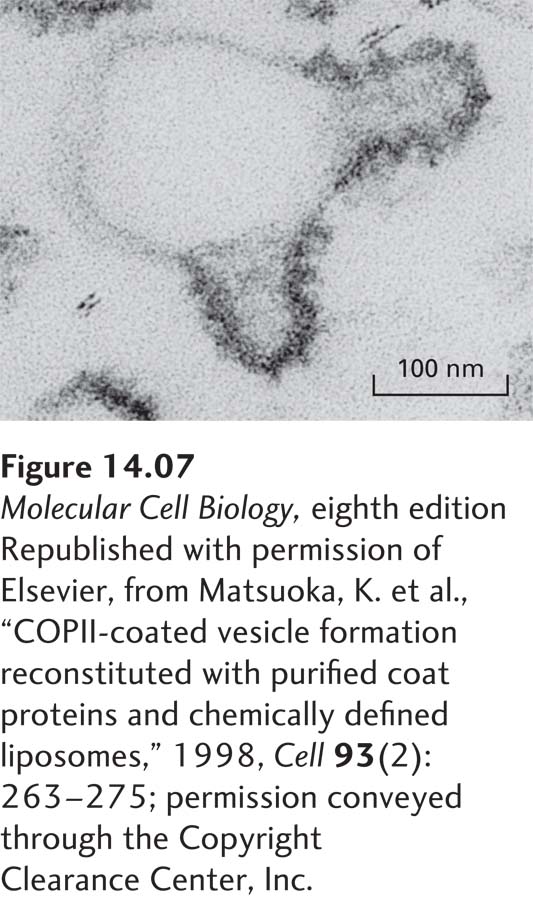
The general scheme of vesicle budding shown in Figure 14-6a applies to all three known types of coated vesicles. Experiments with isolated or artificial membranes and purified coat proteins have shown that polymerization of the coat proteins on the cytosolic face of the parent membrane is necessary to produce the high curvature of the membrane that is typical of a transport vesicle about 50 nm in diameter. Electron micrographs of in vitro budding reactions often reveal structures that exhibit discrete regions of the parent membrane bearing a dense coat accompanied by the curvature characteristic of a completed vesicle (Figure 14-7). Such structures, usually called vesicle buds, appear to be intermediates that are visible after the coat has begun to polymerize but before the completed vesicle pinches off from the parent membrane. The polymerized coat proteins are thought to form a curved lattice that drives the formation of a vesicle bud by adhering to the cytosolic face of the membrane.