A Conserved Set of GTPase Switch Proteins Controls the Assembly of Different Vesicle Coats
Using in vitro vesicle-budding reactions among isolated membranes and purified coat proteins, scientists have determined the minimum set of coat components required to form each of the three major types of vesicles. Although most of the coat proteins differ considerably from one type of vesicle to another, the coats of all three vesicles contain a small GTP-binding protein that acts as a regulatory subunit to control coat assembly (see Figure 14-6a). A GTP-binding protein known as ARF protein plays this role in COPI and clathrin-coated vesicles. A different but related GTP-binding protein known as Sar1 protein is present in the coat of COPII vesicles. Both ARF and Sar1 are monomeric proteins with a structure generally similar to that of Ras, a key intracellular signal-transducing protein (see Figure 16-23). ARF and Sar1 proteins, like Ras, belong to the GTPase superfamily of switch proteins that cycle between GDP-bound and GTP-bound forms (see Figure 3-34 to review the mechanism of GTPase switch proteins).
The cycle of GTP binding and hydrolysis by ARF and Sar1 is thought to control the initiation of coat assembly, as schematically depicted for the assembly of COPII vesicles in Figure 14-8. First, an ER membrane protein known as Sec12 catalyzes the release of GDP from cytosolic Sar1·GDP and the binding of GTP. This guanine nucleotide exchange factor (GEF) apparently receives and integrates multiple as yet unknown signals, probably including the presence in the ER membrane of cargo proteins that are ready to be transported. Binding of GTP causes a conformational change in Sar1 that exposes its amphipathic N-terminus, which then becomes embedded in the phospholipid bilayer and tethers Sar1·GTP to the ER membrane (Figure 14-8, step 1). The membrane-attached Sar1·GTP drives the polymerization of cytosolic complexes of COPII subunits on the membrane, eventually leading to formation of vesicle buds (step 2). Once COPII vesicles are released from the donor membrane, the Sar1 GTPase activity hydrolyzes Sar1·GTP in the vesicle membrane to Sar1·GDP with the assistance of one of the coat subunits (step 3 ). This hydrolysis triggers disassembly of the COPII coat (step 4). Thus Sar1 couples a cycle of GTP binding and hydrolysis to the formation and then dissociation of the COPII coat.
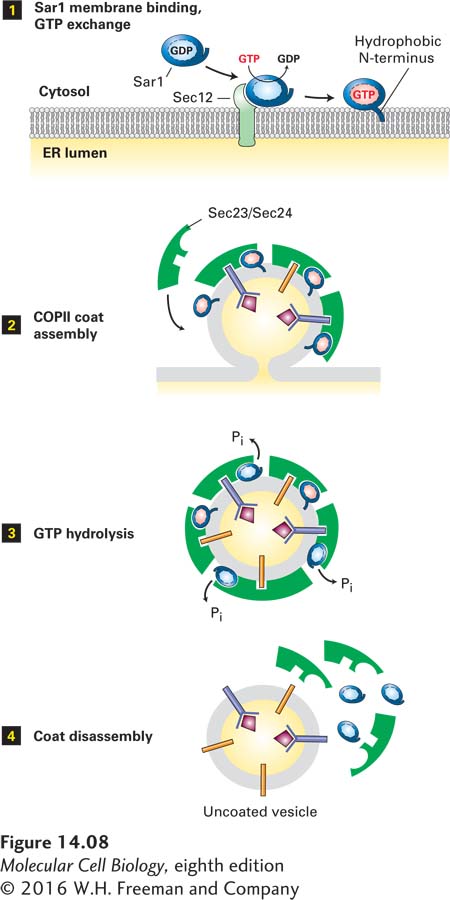
FIGURE 14-8 Model for the role of Sar1 in the assembly and disassembly of the COPII coat. Step 1: Interaction of soluble GDP-bound Sar1 with the GEF Sec12, an ER integral membrane protein, catalyzes exchange of GTP for GDP on Sar1. The hydrophobic N-terminus of the GTP-bound form of Sar1 extends outward from the protein’s surface and anchors Sar1 to the ER membrane. Step 2: Sar1 attached to the membrane serves as a binding site for the Sec23/Sec24 coat protein complex. Membrane cargo proteins are recruited to the forming vesicle bud by binding of specific short sequences (sorting signals) in their cytosolic regions to sites on the Sec23/Sec24 complex. Some membrane cargo proteins also act as receptors that bind soluble proteins in the lumen. The coat is completed by assembly of a second type of coat complex composed of Sec13 and Sec31 (not shown). Step 3: After the vesicle coat is complete, the Sec23 coat subunit promotes GTP hydrolysis by Sar1. Step 4: Release of Sar1·GDP from the vesicle membrane causes disassembly of the coat. See S. Springer et al., 1999, Cell 97:145.
ARF protein undergoes a similar cycle of nucleotide exchange and hydrolysis coupled to the assembly of vesicle coats composed either of COPI or of clathrin and other coat proteins (AP complexes), discussed later. A covalent protein modification known as a myristate anchor on the N-terminus of the ARF protein weakly tethers ARF·GDP to the Golgi membrane. When GTP is exchanged for the bound GDP by a GEF attached to the Golgi membrane, the resulting conformational change in ARF allows hydrophobic residues in its N-terminal segment to insert into the membrane bilayer. The resulting tight association of ARF·GTP with the membrane serves as the foundation for further coat assembly.
Drawing on the structural similarities of Sar1 and ARF to other small GTPase switch proteins, researchers have constructed genes encoding mutant versions of the two proteins that have predictable effects on vesicular traffic when transfected into cultured cells. For example, in cells expressing mutant versions of Sar1 or ARF that cannot hydrolyze GTP, vesicle coats form and vesicle buds pinch off. However, because the mutant proteins cannot trigger disassembly of the coat, all available coat subunits eventually become permanently assembled into coated vesicles that are unable to fuse with target membranes. Addition of a nonhydrolyzable GTP analog to in vitro vesicle-budding reactions causes a similar blocking of coat disassembly. The vesicles that form in such reactions have coats that never dissociate, allowing their composition and structure to be more readily analyzed. The purified COPI vesicles shown in Figure 14-9 were produced in such a budding reaction.
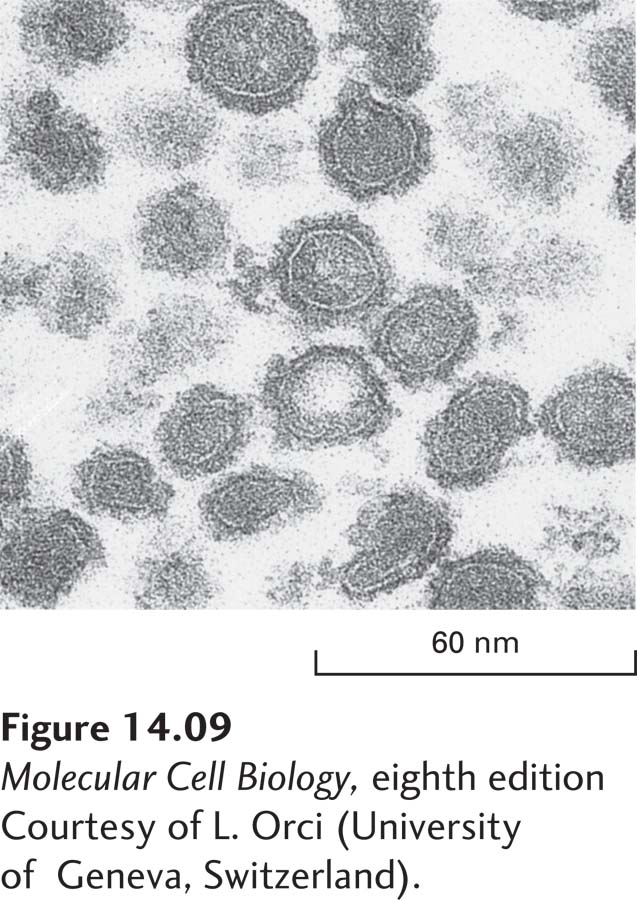
EXPERIMENTAL FIGURE 14-9 Coated vesicles accumulate during in vitro budding reactions in the presence of a nonhydrolyzable analog of GTP. When isolated Golgi membranes are incubated with a cytosolic extract containing COPI coat proteins, vesicles form and bud off from the membranes. Inclusion of a nonhydrolyzable analog of GTP in the budding reaction prevents disassembly of the coat after vesicle release. This micrograph shows COPI vesicles generated in such a reaction and separated from membranes by centrifugation. Coated vesicles prepared in this way can be analyzed to determine their components and properties.
[Courtesy of L. Orci (University of Geneva, Switzerland).]
A second general function of small GTPases in vesicle formation is the pinching off of a completed vesicle from the parent membrane. In vitro budding experiments show that the Sar1 GTPase is required for the pinching off of COPII vesicles and that the ARF GTPase drives the pinching off of COPI vesicles. The mechanism by which these small GTPases convert the energy from GTP hydrolysis to a mechanical force to complete the pinching off of the membrane is not understood. As we will see in Section 14.4, a large polymeric GTPase known as dynamin plays this role in clathrin-coated vesicles.

