Rod Cells Adapt to Varying Levels of Ambient Light by Intracellular Trafficking of Arrestin and Transducin
Whereas cone cells are insensitive to low levels of light, rod-cell function is saturated by high levels of light. When we move from bright sunlight into a dimly lit room, we initially cannot see the objects around us. The reason is because it takes some time for our rod cells to become sensitized to the low light level in the room; gradually, we are able to see and distinguish objects. During this time interval, our rod cells “turn up” their sensitivity to light. On the other hand, when we move quickly from a dimly lit room out into bright sunshine, the opposite occurs: our rod cells “turn down” their sensitivity to light. As the result of this process, called visual adaptation, rod cells are able to perceive light contrast over a 100,000-fold range of ambient light levels, all the way from a dimly lit room to bright sunlight. This wide range of sensitivities is possible because it is the difference in light levels in the visual field, rather than the absolute amount of light absorbed, that is ultimately sensed by the brain and used to form visual images. As we will see below, it is the subcellular trafficking of two key proteins in the signaling pathway, namely transducin and arrestin, that contribute to the extraordinary range of sensitivity of rod cells.
In dark-adapted rod cells, 80–90 percent of the Gαt and Gβγ transducin subunits are in the outer segments, while fewer than 10 percent of arrestin molecules are localized there (Figure 15-22). This spatial distribution allows maximal activation of the downstream effector PDE and thus maximal sensitivity to small changes in light. But exposure for 10 minutes to moderate daytime intensities of light causes a complete redistribution of these proteins: over 80 percent of the Gαt and Gβγ subunits move out of the outer segment into other parts of the cell, while over 80 percent of the inhibitor arrestin moves into the outer segment.
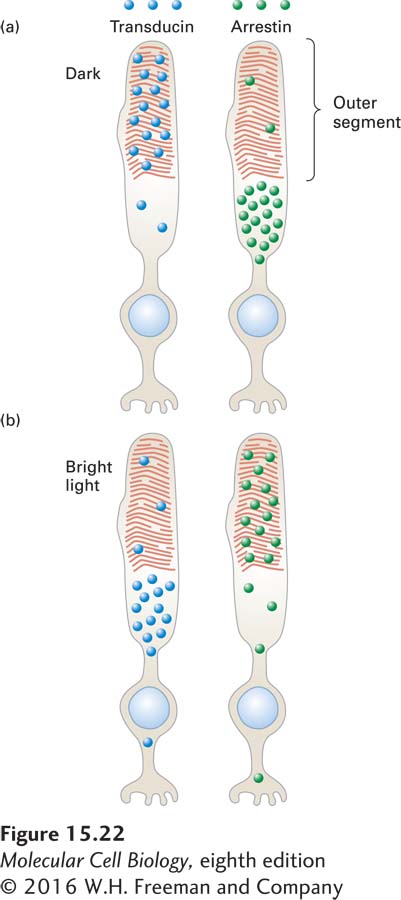
FIGURE 15-22 Schematic illustration of transducin and arrestin distribution in dark-adapted and light-adapted rod cells. (a) In the dark, most transducin (blue circles) is localized to the outer segment, while most arrestin (green circles) is found in other parts of the cell; in this condition, vision is most sensitive to very low light levels. (b) In bright light, little transducin is found in the outer segment, and abundant arrestin is found there; in this condition, vision is relatively insensitive to small changes in light. The coordinated movement of these proteins contributes to our ability to perceive images over a 100,000-fold range of ambient light levels. See P. Calvert et al., 2006, Trends Cell Biol. 16:560.
The mechanism by which these proteins move within the cell is not yet known, but it probably involves microtubule-attached motors that carry proteins and other cargos into and out of different subcellular regions (see Chapter 18). In bright light, the reduction in the level of transducin, with its Gαt and Gβγ subunits, in the outer segment means that Gαt is simply not available for binding to activated rhodopsin. As a result, less PDE is activated. At the same time, the increase in the arrestin level in the outer segment means that any activated rhodopsin that is present will become rapidly inactivated. Together, the drop in transducin level and the increase in arrestin level greatly reduce the ability of small increases in light levels to activate the downstream effector PDE; thus only large changes in light levels will be sensed by the rod cells. These protein movements are reversed when the ambient light level is lowered.
