15.5 G Protein–Coupled Receptors That Activate or Inhibit Adenylyl Cyclase
GPCR signal transduction pathways that use adenylyl cyclase as an effector protein and cAMP as a second messenger are found in most mammalian cells, where they regulate cellular functions as diverse as metabolism of fats and sugars, synthesis and secretion of hormones, and muscle contraction. These pathways follow the general GPCR mechanism outlined in Figure 15-14: ligand binding to the receptor activates a coupled heterotrimeric G protein, termed Gαs, that activates an effector protein—in this case, adenylyl cyclase, which synthesizes the diffusible second messenger cAMP from ATP (Figure 15-23). The cAMP, in turn, activates a cAMP-dependent protein kinase that phosphorylates specific target proteins. In mammals, more than 30 different GPCRs activate Gαs and adenylyl cyclase; most cell types express one or more such GPCRs. As detailed in Classic Experiment 15-1, early studies of the mechanism of activation of adenylyl cyclase led to the discovery of the role of the first GTP-binding protein in receptor signaling.
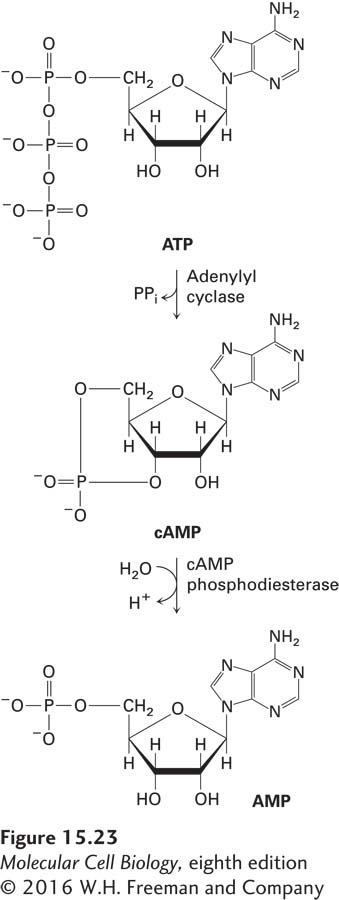
FIGURE 15-23 Synthesis and hydrolysis of cAMP by adenylyl cyclase and PDE. Similar reactions occur for production of cGMP from GTP and hydrolysis of cGMP.
To explore this GPCR/cAMP pathway, we focus on the first such pathway discovered: the hormone-stimulated generation of glucose-1-phosphate from glycogen, a storage polymer of glucose (Figure 15-24). The breakdown of glycogen (glycogenolysis), which occurs in muscle and liver cells in response to hormones such as epinephrine and glucagon, is a principal way in which glucose is made available to cells in need of energy. This example shows how activation of a GPCR can stimulate or inhibit several intracellular enzymes, all coordinated to carry out a physiologically important task: glycogen metabolism.
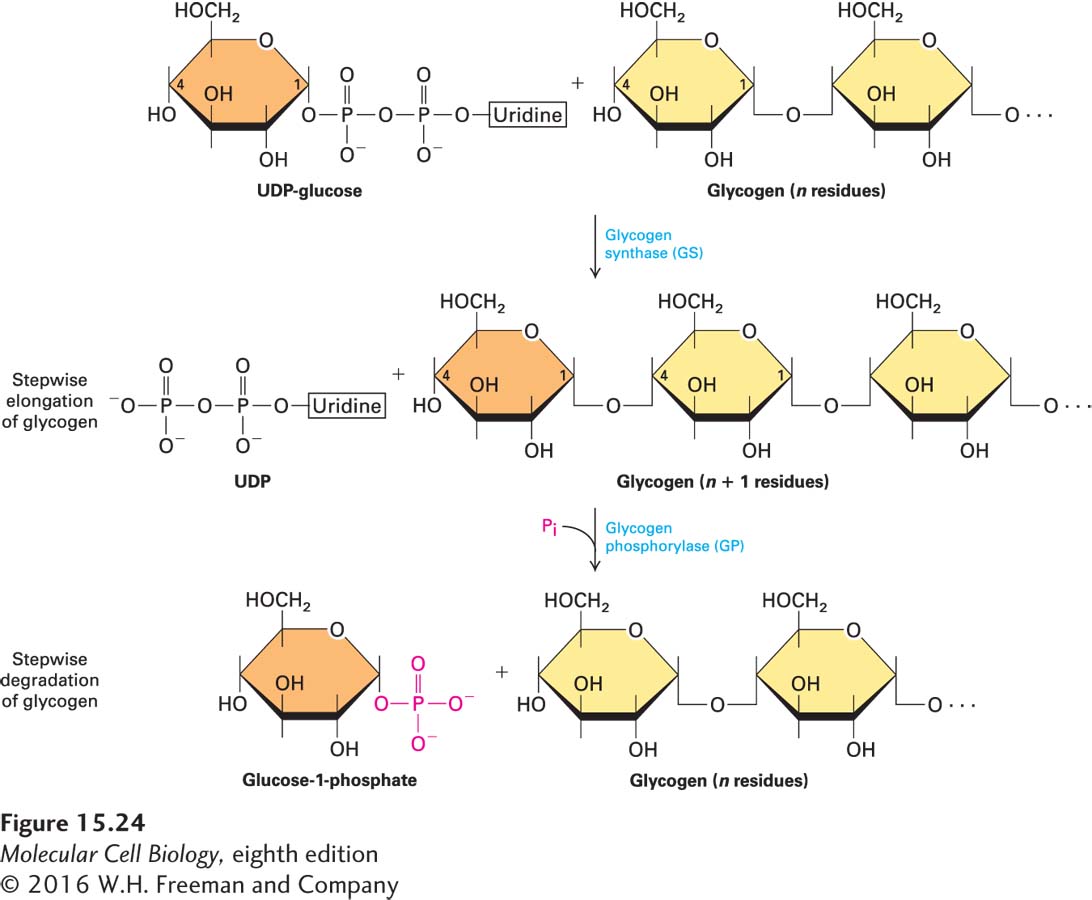
FIGURE 15-24 Synthesis and degradation of glycogen. Incorporation of glucose from UDP-glucose into glycogen is catalyzed by glycogen synthase. Removal of glucose units from glycogen is catalyzed by glycogen phosphorylase. Because two different enzymes catalyze the formation and the degradation of glycogen, the two reactions can be independently regulated.

