Glycogen Metabolism Is Regulated by Hormone-Induced Activation of PKA
Like all biopolymers, glycogen is synthesized by one set of enzymes and degraded by another (see Figure 15-24). Degradation of glycogen, or glycogenolysis, involves the stepwise removal of glucose residues from one end of the polymer by a phosphorolysis reaction, catalyzed by glycogen phosphorylase (GP), yielding glucose-1-phosphate.
In both muscle and liver cells, glucose-1-phosphate produced from glycogen is converted by an enzyme to glucose-6-phosphate. In muscle cells, this metabolite enters the glycolytic pathway and is metabolized to generate ATP for use in powering muscle contraction (see Chapters 12 and 17). Unlike muscle cells, liver cells contain a phosphatase that hydrolyzes glucose-6-phosphate to glucose, which is exported from these cells mainly by a glucose transporter (GLUT2) in the plasma membrane (see Chapter 11). Thus glycogen stores in the liver are primarily broken down to glucose, which is immediately released into the blood and transported to other tissues, particularly the muscles and brain, to nourish them. Glycogenolysis in both types of cells is induced by rises in blood epinephrine as part of the fight-or-flight response.
Activated PKA enhances the conversion of glycogen to glucose-1-phosphate in two ways: by inhibiting glycogen synthesis and by stimulating glycogen degradation (Figure 15-28a). PKA directly phosphorylates and, in so doing, inactivates glycogen synthase (GS), the enzyme that synthesizes glycogen. PKA promotes glycogen degradation indirectly by phosphorylating and thus activating an intermediate kinase, glycogen phosphorylase kinase (GPK). In turn, active GPK phosphorylates and activates GP, the enzyme that degrades glycogen.
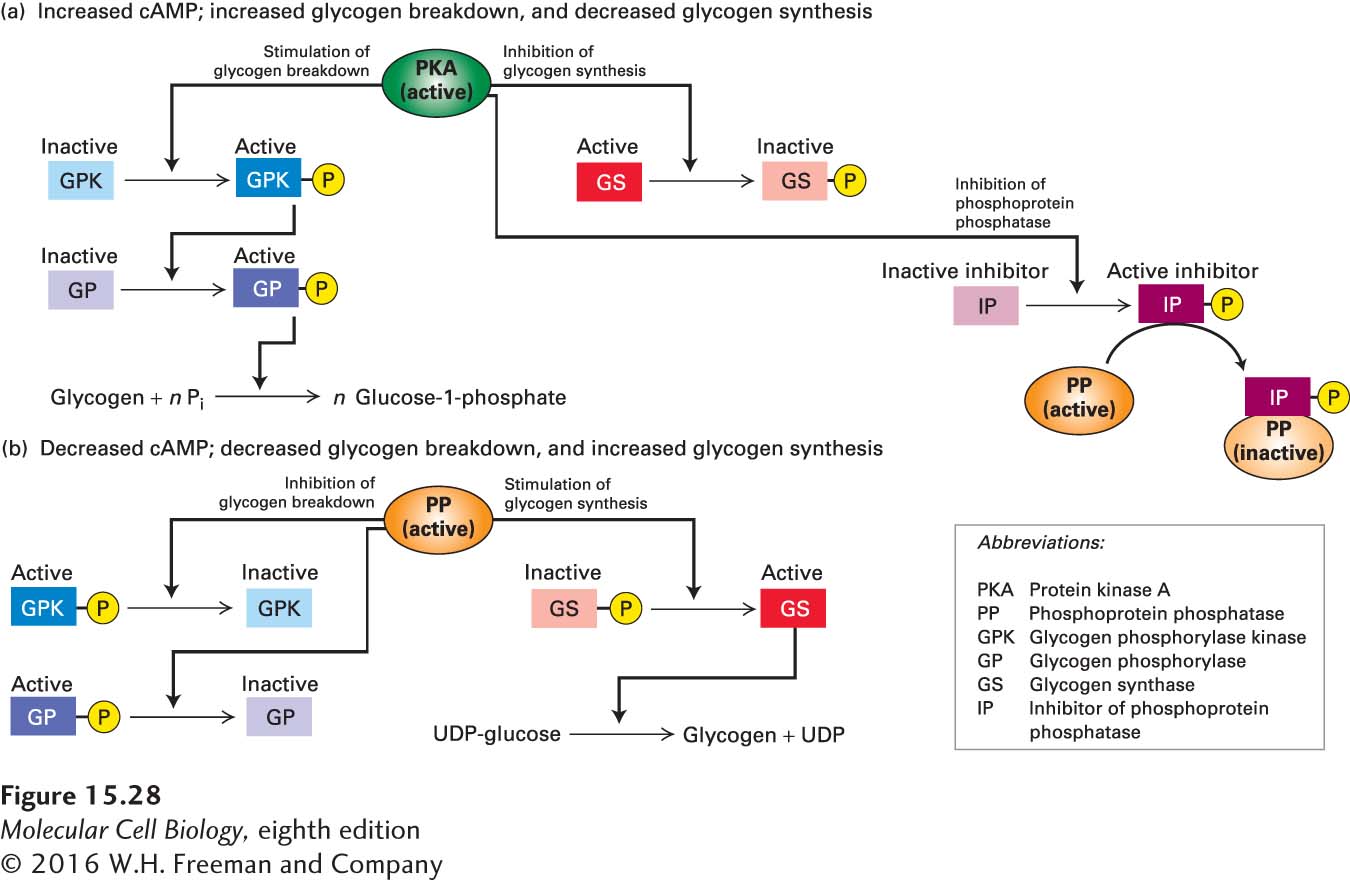
FIGURE 15-28 Regulation of glycogen metabolism by cAMP and PKA. Active enzymes are highlighted in darker shades; inactive forms, in lighter shades. (a) An increase in cytosolic cAMP activates PKA, which phosphorylates glycogen synthase (GS) and thus inhibits glycogen synthesis directly. Active PKA also promotes glycogen degradation via a protein kinase cascade. At high cAMP concentrations, PKA also phosphorylates an inhibitor of phosphoprotein phosphatase (PP). Binding of the phosphorylated inhibitor to PP prevents this phosphatase from de-phosphorylating the activated enzymes in the kinase cascade or the inactive glycogen synthase. (b) A decrease in cAMP inactivates PKA, leading to release of the active form of PP. The activation of PP promotes glycogen synthesis and inhibits glycogen degradation.
Skeletal muscle GPK is a huge protein of subunit composition (αβγδ)4. The γ subunit contains the kinase catalytic activity and the others are regulatory; the δ subunit is the ubiquitous protein calmodulin, which has four calcium ion binding sites (see Figure 3-33). GPK enzyme activity is increased both by phosphorylation of the α and β subunits by PKA and by Ca2+ binding to the δ subunit; maximal activity requires both stimulators.
All three enzymes—GS, GPK, and GP—are counteracted by a phosphatase called phosphoprotein phosphatase (PP). At high cAMP levels, PKA phosphorylates an inhibitor of phosphoprotein phosphatase (IP), which keeps this phosphatase in its inactive state (see Figure 15-28a, right).
The entire process is reversed when epinephrine or another hormone activating Gαs is removed and the level of cAMP drops, inactivating PKA. When PKA is inactive, it can no longer phosphorylate IP, so PP becomes active (Figure 15-28b). PP removes the phosphate residues previously added by PKA to GS and GPK, as well as the phosphates on GP added by GPK. As a consequence, the synthesis of glycogen by GS is enhanced and the degradation of glycogen by GP is inhibited.
Epinephrine-induced glycogenolysis thus exhibits dual regulation: activation of the enzymes catalyzing glycogen degradation and inhibition of enzymes promoting glycogen synthesis. Such dual regulation provides an efficient mechanism for regulating a particular cellular response and is a common phenomenon in cell biology.
