Integration of Ca2+ and cAMP Second Messengers Regulates Glycogenolysis
All cells constantly receive multiple signals from their environment, including changes in levels of hormones, metabolites, and gases such as NO and oxygen. All of these signals must be integrated. The breakdown of glycogen to glucose (glycogenolysis) provides an excellent example of how cells can integrate their responses to more than one signal. As discussed in Section 15.5, epinephrine stimulation of muscle and liver cells leads to a rise in the second messenger cAMP, which promotes glycogen breakdown (see Figure 15-28a). In both muscle and liver cells, other signaling pathways produce the same cellular response of enhanced glycogenolysis.
In striated muscle cells (see Figure 17-30), stimulation by nerve impulses causes the release of Ca2+ ions from the sarcoplasmic reticulum and an increase in the cytosolic Ca2+ concentration, which triggers muscle contraction. The rise in cytosolic Ca2+ also allows Ca2+ binding to the δ subunit of glycogen phosphorylase kinase (GPK)—which is calmodulin—
Rises in blood epinephrine concentrations lead to activation of adenylyl cyclase and an increase in cAMP concentrations. Recall that phosphorylation by cAMP-
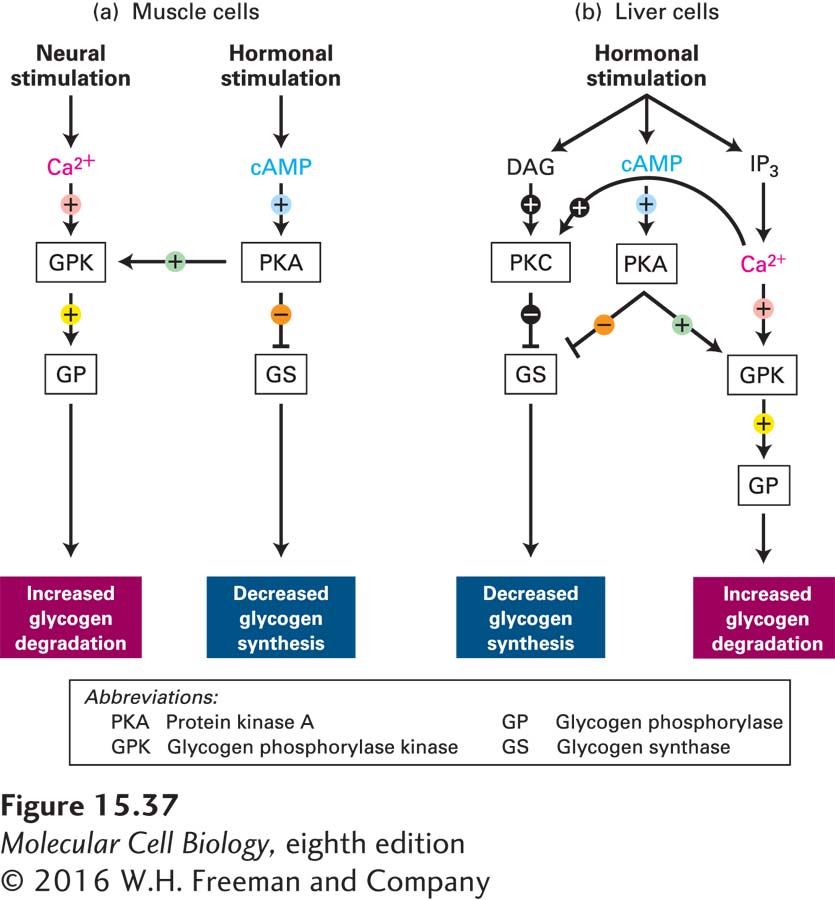
In liver cells, hormone-
Recall that GPK is maximally active when Ca2+ ions are bound to the δ (calmodulin) subunit and the α subunit has been phosphorylated by PKA. After neuronal stimulation of muscle cells, GPK is sufficiently active, even if it is nonphosphorylated, to allow degradation of glycogen in the absence of hormone stimulation. Binding of Ca2+ to the δ subunit may be essential to the enzymatic activity of GPK. Phosphorylation of the α and also the β subunits by PKA increases the affinity of the δ subunit for Ca2+, allowing Ca2+ ions to bind to the enzyme at the submicromolar Ca2+ concentrations found in resting cells.