Protein Kinases and Phosphatases Are Employed in Many Signaling Pathways
Activation of virtually all cell-
677
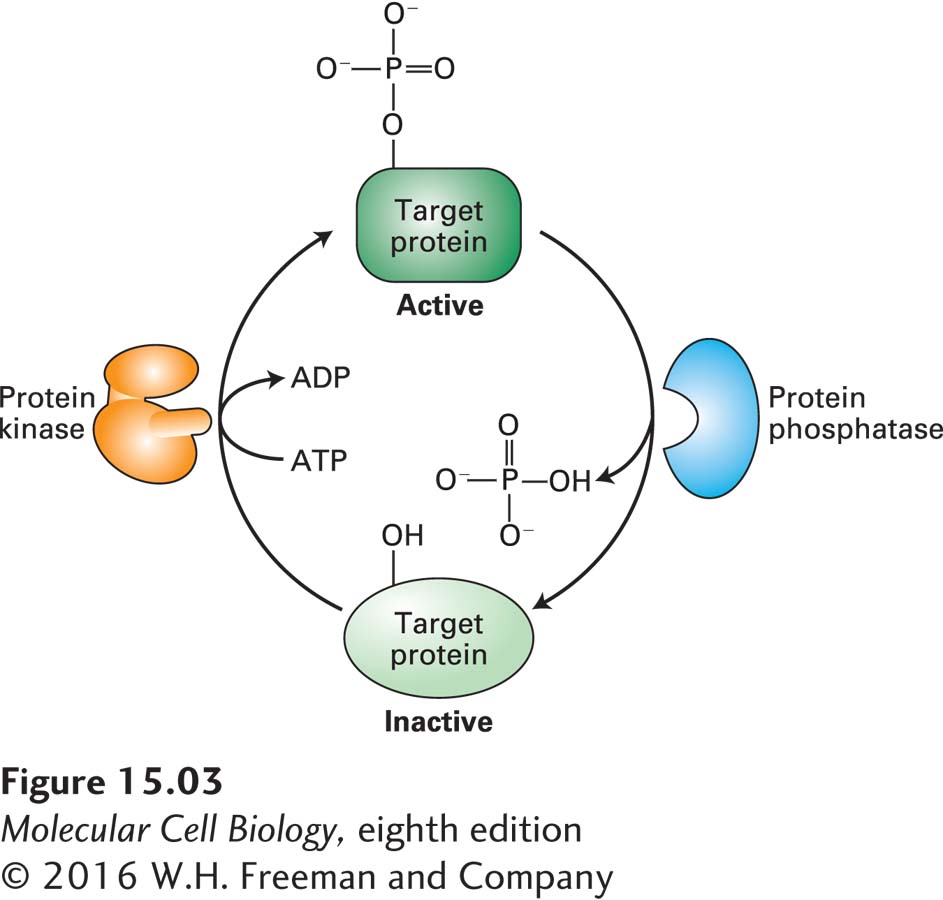
The human genome encodes about 600 protein kinases and 100 different phosphatases. In general, each protein kinase phosphorylates specific amino acid residues in a specific set of target, or substrate, proteins whose patterns of expression generally differ in different cell types. Animal cells contain two types of protein kinases: those that add phosphate to the hydroxyl group on tyrosine residues (protein tyrosine kinases) and those that add phosphate to the hydroxyl group on serine or threonine (or both) residues (protein serine/threonine kinases). We will see in this chapter and the next that the catalytic subunits of all known protein kinases have similar three-
Many proteins are substrates for multiple kinases, each of which usually phosphorylates different amino acids in the protein. Each phosphorylation event has the potential to modify the activity of a particular target protein in different ways, some activating its function, others inhibiting it. An example we will encounter later is glycogen phosphorylase kinase, a key regulatory enzyme in glucose metabolism (see Figure 15-29). In many cases, addition of a phosphate group to an amino acid creates a binding surface that allows a second protein to bind; in the following chapter, we will encounter many examples of such kinase-
Commonly the catalytic activity of a protein kinase itself is modulated by phosphorylation by other kinases, by the binding of other proteins to it, and by changes in the concentrations of various small intracellular signaling molecules and metabolites. Importantly, the activity of all protein kinases is opposed by the activity of protein phosphatases, some of which themselves are regulated by extracellular signals. Thus the activity of a protein in a cell can be a complex function of the activities of the usually multiple kinases and phosphatases that act on it. Several examples of this phenomenon that occur in regulation of the cell cycle are described in Chapter 19.