Intracellular “Second Messengers” Transmit Signals from Many Receptors
The binding of ligands (“first messengers”) to many cell-surface receptors leads to a short-lived increase (or decrease) in the concentration of certain nonprotein, low-molecular-weight intracellular signaling molecules termed second messengers. These molecules, in turn, bind to proteins, modifying their activity.
One second messenger used in virtually all metazoan cells is calcium (Ca2+) ions. We noted in Chapter 11 that the concentration of free Ca2+ in the cytosol is kept very low (~10−7 M) in part by ATP-powered pumps that continually transport Ca2+ out of the cell or into the endoplasmic reticulum (ER). The cytosolic Ca2+ concentration can be increased from tenfold to a hundredfold by a signal-induced release of Ca2+ from the ER lumen or the extracellular environment by the opening of calcium channels in the respective membranes; this change can be detected by fluorescent dyes introduced into the cell (see Figure 4-12). In muscle, a signal-induced rise in cytosolic Ca2+ triggers contraction (see Figure 17-34). In endocrine cells, a similar increase in Ca2+ induces exocytosis of secretory vesicles containing hormones, which are thus released into the circulation. In neurons, an increase in cytosolic Ca2+ leads to the exocytosis of neurotransmitter-containing vesicles (see Chapter 22). In all cells, such a rise in cytosolic Ca2+ is sensed by Ca2+-binding proteins, particularly those of the EF hand family, such as calmodulin, all of which contain the helix-loop-helix motif (see Figure 3-10b). The binding of Ca2+ to calmodulin and other EF hand proteins causes a conformational change that permits those proteins to bind various target proteins, thereby switching their activities on or off (see Figure 3-33). Often a rise in Ca2+ is localized to specific regions of the cytosol, allowing a process—such as exocytosis of a secretory vesicle—to occur there without affecting other processes elsewhere in the cell.
Another nearly universal second messenger is cyclic adenosine monophosphate (cAMP). In many eukaryotic cells, a rise in cAMP triggers the activation of a particular protein kinase, protein kinase A, that in turn phosphorylates specific target proteins to induce specific changes in cell metabolism. In some cells, cAMP regulates the activity of certain ion channels. The structures of cAMP and three other common second messengers are shown in Figure 15-6. Later in this chapter, we examine the specific roles of second messengers in signaling pathways activated by various G protein–coupled receptors.
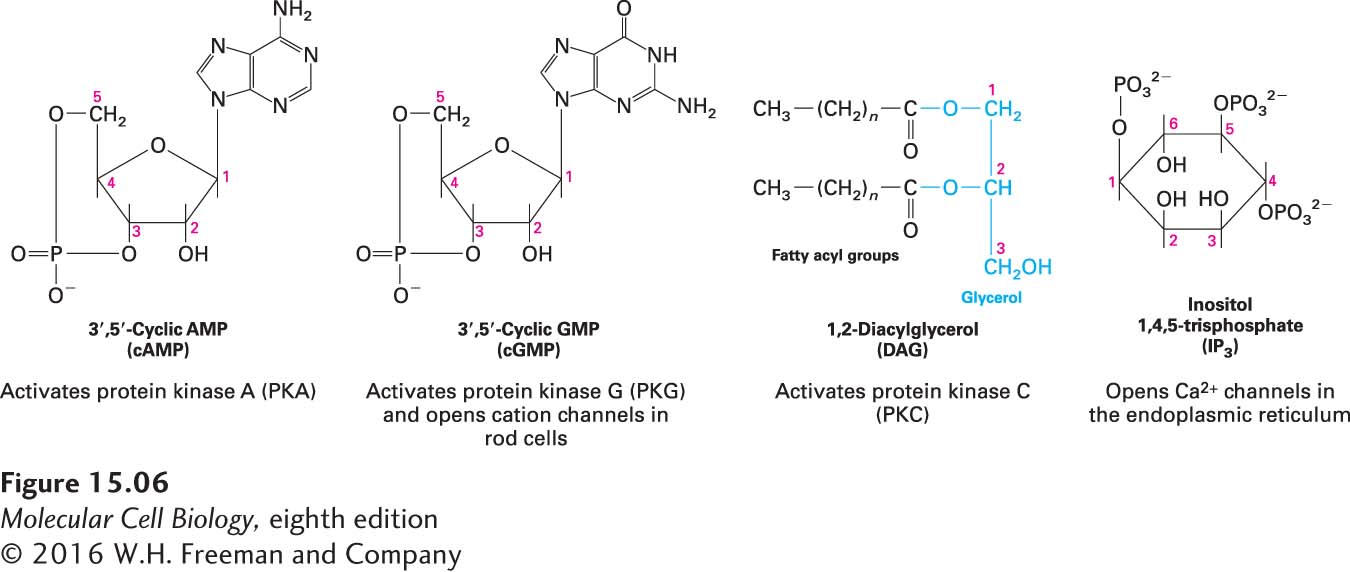
FIGURE 15-6 Four common intracellular second messengers. The major direct effect or effects of each compound are indicated below its structural formula. Calcium ions (Ca2+) and several membrane-bound phosphatidylinositol derivatives also act as second messengers.
