Chapter Introduction
Signaling Pathways That Control Gene Expression
A molecular valentine—dimerized extracellular domain of the epidermal growth factor receptor (red) bound to two molecules of epidermal growth factor (pink).
[Data from H. Ogiso et al., 2002, Cell 110:775, PDB ID 1ivo.]
OUTLINE
16.1 Receptor Serine Kinases That Activate Smads
16.2 Cytokine Receptors and the JAK/STAT Signaling Pathway
16.3 Receptor Tyrosine Kinases
16.4 The Ras/MAP Kinase Pathway
16.5 Phosphoinositide Signaling Pathways
16.6 Signaling Pathways Controlled by Ubiquitinylation and Protein Degradation: Wnt, Hedgehog, and NF-κB
16.7 Signaling Pathways Controlled by Protein Cleavage: Notch/Delta, SREBP, and Alzheimer’s Disease
16.8 Integration of Cellular Responses to Multiple Signaling Pathways: Insulin Action
Extracellular signals can have both short- and long-term effects on cells. Short-term effects are usually triggered by modification of existing enzymes or other proteins, as we saw in Chapter 15. Many extracellular signals also affect gene expression and thus induce long-term changes in cell function. These changes include alterations in cell division and development, such as those that occur during cell fate determination and differentiation. The body’s production of red blood cells, white blood cells, and platelets in response to the cytokines we discuss later in this chapter is a good example of signal-induced changes in gene expression that influence cell proliferation and differentiation. Changes in gene expression also enable differentiated cells to respond to their environment by changing their shape, metabolism, or movement. In immune-system cells, for example, several hormones activate one type of transcription factor (NF-κB) that ultimately affects the expression of more than 150 genes involved in the immune response to infection. Given the extensive role of gene transcription in mediating critical aspects of development, metabolism, and movement, it is not surprising that mutations in such signaling pathways cause many human diseases, including cancer, diabetes, and immune-system disorders.
In this chapter, we explore the main signaling pathways that cells use to influence gene expression. In eukaryotes, there are about a dozen classes of highly conserved cell-surface receptors, and these receptors activate several types of highly conserved intracellular signal transduction pathways. Many of these pathways consist of multiple kinases, GTP-binding proteins, other regulatory proteins, small intracellular molecules, and ions such as Ca2+, which together form a complex signaling cascade. Given this complexity, cell signaling can seem a daunting subject to learn for the first time; the many names and abbreviations of the molecules found in each pathway can indeed be challenging. The subject repays careful study, however: when one becomes familiar with these pathways, one understands in a profound way the regulatory mechanisms that control a vast array of biological processes.
For simplicity, signal transduction pathways can be grouped into several basic types, based on the sequence of intracellular events. In one very common type of signal transduction pathway (Figure 16-1a), the binding of a ligand to a receptor triggers activation of a receptor-associated kinase. These receptors generally have a single transmembrane domain and are activated by ligand-induced receptor dimerization. The kinase may be an intrinsic part of the cytosolic domain of the receptor protein or may be tightly bound to the cytosolic domain of the receptor. These kinases often directly phosphorylate and activate a variety of signal-transducing proteins, including transcription factors located in the cytosol (Figure 16-1a, step 1). Some receptor kinases also activate small GTP-binding “switch” proteins such as Ras (Figure 16-1a, step 2). Many signal transduction pathways, such as those activated by Ras, involve several kinases in which one kinase phosphorylates and thus activates (or occasionally inhibits) the activity of another kinase; one or more of these kinases eventually phosphorylate and activate transcription factors.
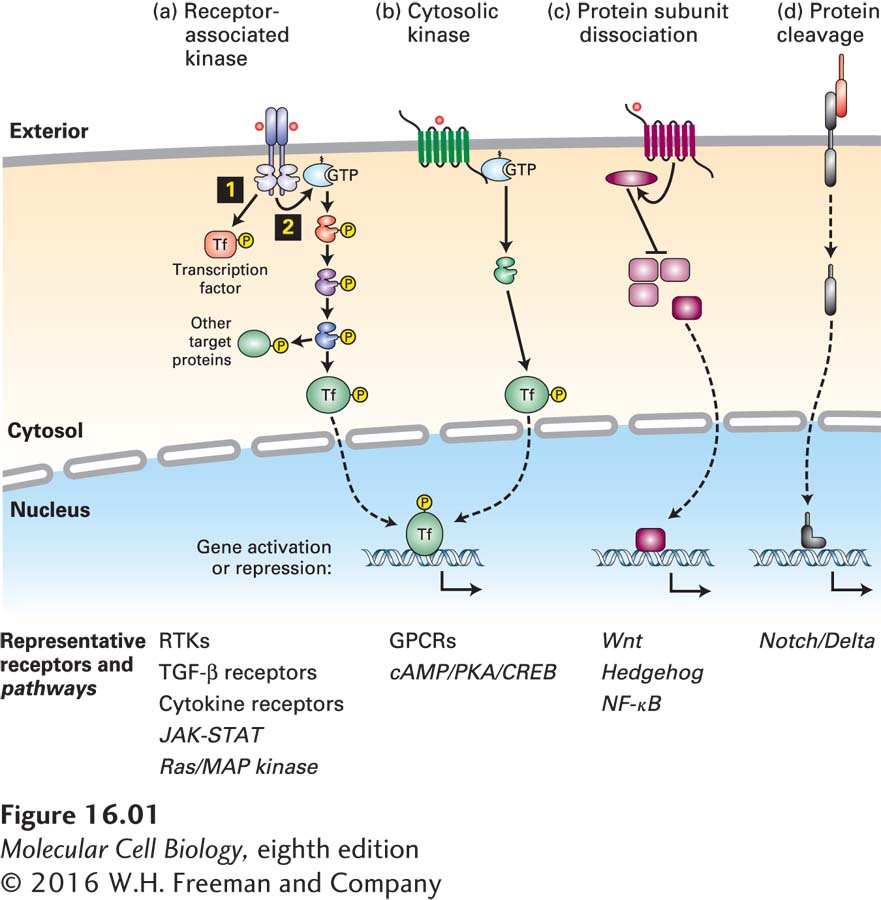
FIGURE 16-1 Several common types of cell-surface receptors and signal transduction pathways. (a) The cytosolic domains of many receptors are protein kinases or are tightly associated with a cytosolic kinase; commonly the kinases are activated by ligand binding followed by receptor dimerization. Some of these kinases directly phosphorylate and activate transcription factors 1 or other signaling proteins. Many of these receptors also activate small GTP-binding “switch” proteins such as Ras 2. Many signal transduction pathways, such as those activated by Ras, involve several kinases; in these pathways, one kinase phosphorylates and thus activates (or occasionally inhibits) the activity of another kinase. Many of the kinases in these pathways phosphorylate multiple protein targets, including transcription factors, which are usually different in different cells. (b) Other receptors, mainly those with seven membrane-spanning segments, activate the larger GTP-binding Gα proteins, which in turn activate specific kinases or other signaling proteins. (c) Several signaling pathways involve disassembly of a multiprotein complex in the cytosol, releasing a transcription factor that then translocates into the nucleus. (d) Some signaling pathways are irreversible; in many cases, proteolytic cleavage of a receptor releases an active transcription factor.
Most other receptors become activated by conformational changes induced by ligand binding. Some, mainly the receptors with seven membrane-spanning segments introduced in Chapter 15, activate GTP-binding Gα proteins (Figure 16-1b). Their activation usually leads to activation of one or more protein kinases, which in turn phosphorylate and activate multiple target proteins, including transcription factors.
In yet other signaling pathways, binding of a ligand to a receptor triggers disassembly of a multiprotein complex in the cytosol, releasing a transcription factor that then translocates into the nucleus and affects gene expression (Figure 16-1c). Finally, in the last common type, proteolytic cleavage of an inhibitor or of the receptor itself releases an active transcription factor, which then travels into the nucleus (Figure 16-1d). While every signaling pathway has its own subtleties and distinctions, nearly every one can be grouped into one of these basic types.
The pathways we discuss in this chapter have been conserved throughout evolution and operate in much the same manner in flies, worms, planaria, and humans. The substantial homology exhibited among proteins in these pathways has enabled researchers to study them in a variety of experimental systems. For instance, the secreted signaling protein Hedgehog (Hh) and its receptor were first identified in Drosophila mutants that had impaired development. Subsequently, the human and mouse homologs of these proteins were cloned and shown to participate in a number of important signaling events during cell differentiation, resulting in the discovery that abnormal activation of the Hh pathway occurs in several human tumors. Such discoveries illustrate the importance of studying signaling pathways both genetically—in flies, mice, worms, yeasts, and other organisms—and biochemically.
Many receptors are expressed in multiple types of body cells, but activation of these receptors by the same hormone triggers induction (or repression) of very different sets of genes in each cell type. In other words, the same activated transcription factor will induce (or repress) the expression of different sets of genes in different types of cells. To understand this key point, recall from Chapter 9 that the expression of every gene in higher organisms is regulated by multiple transcription factors and chromatin-modifying enzymes, and that many genes are expressed in each of multiple types of cells at very precise but often different levels.
Whether a transcription factor activated by a cell-surface receptor induces (or represses) a gene in a particular cell depends, first, on the epigenetic state of the cell determined by its developmental history (Figure 16-2). As we learned in Chapter 9, the epigenetic state dictates whether a gene is in an active “open” chromatin conformation, and therefore accessible to binding by the transcription factor, or in a silenced “closed” state that is not accessible for transcriptional regulation. In other words, a given transcription factor can potentially bind to multiple gene regulatory sites in chromosomal DNA, but in any given cell type only a fraction of these sites will be accessible for binding.
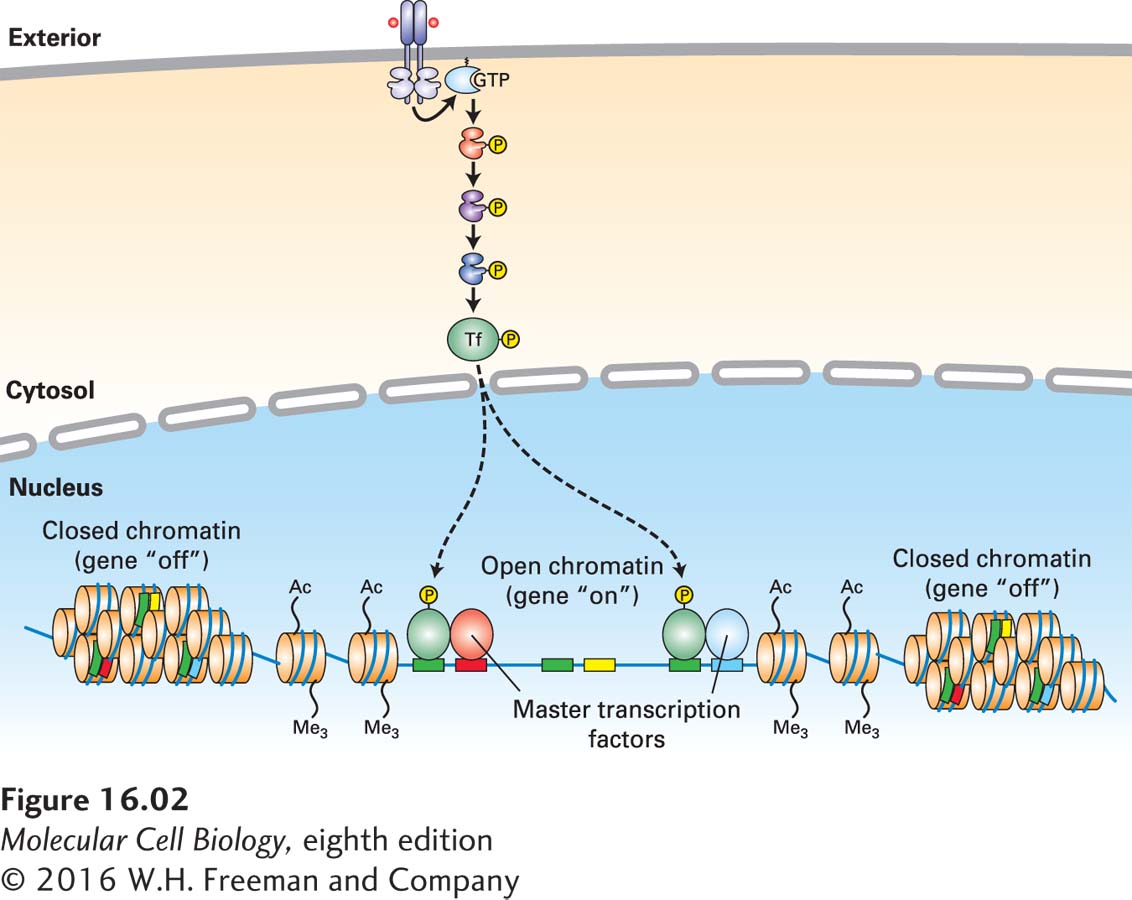
FIGURE 16-2 Induction of a particular gene by a transcription factor depends not only on binding sites for the factor, but also on the gene’s epigenetic state and on the presence of master transcription factors and other nuclear proteins. Any given activated transcription factor has multiple sites on the chromosomal DNA to which it can potentially bind (green), but in any given cell it will bind only to those sites that are in an “open chromatin” conformation and in which specific master transcription factors or other cell-specific proteins (here colored blue and red, respectively) are bound to adjacent sites on the DNA. Other potential transcription factor binding sites are adjacent to binding sites for other master transcription factors (yellow) that are not expressed in this cell type, and thus the transcription factor will not bind to those sites.
Second, other transcription factors, histone readers and modifiers, and chromatin remodelers that interact with the particular activated transcription factor determine what genes will be induced or repressed by the binding of that factor to gene regulatory sequences. In particular, many cell types express one or more master transcription factors that determine the identity and developmental fate of the cell; a recent finding is that many transcription factors activated by cell-surface receptors bind to chromosomal DNA at regulatory sites—mainly enhancers—adjacent to these master factors and thus induce (or repress) cell-specific genes (see Figure 16-2).
No signaling pathway acts in isolation. Many cells respond to multiple types of hormones and other signaling molecules; some mammalian cells express roughly a hundred different types of cell-surface receptors, each of which binds a different ligand. Since many genes are regulated by multiple transcription factors, which in turn are activated or repressed by different intracellular signaling pathways, expression of any one gene can be regulated by multiple extracellular signals. Especially during early development, such “cross talk” between signaling pathways and the resultant sequential alterations in the pattern of gene expression eventually can become so extensive that the cell assumes a different developmental fate. In this chapter, we will see how multiple signaling pathways interact to regulate crucial aspects of metabolism, such as the level of glucose in the blood and the formation of adipose (fat-storing) cells.
We begin with a discussion of a large class of receptors—the receptor serine kinases—that, when activated by their corresponding hormones, directly phosphorylate and activate one or more transcription factors that move directly into the nucleus. We will see precisely how these transcription factors activate very different genes in different cell types depending on the epigenetic state of the chromatin and the presence of master transcription factors and other cell-specific proteins.


