Binding of a Cytokine to Its Receptor Activates One or More Tightly Bound JAK Protein Tyrosine Kinases
GH, prolactin, G-CSF, thrombopoietin, and Epo all have similar structures, and they activate receptors of similar structure by forming dimers of two identical cytokine receptor proteins, a process termed receptor homodimerization. Each of these receptor proteins then activates JAK2, the JAK kinase to which it is bound. The extracellular domains of these cytokine receptors are constructed of two subdomains, each of which contains seven conserved β strands folded together in a characteristic fashion.
Many other cytokines, including the interleukins that activate immune-system cells and the interferons that induce expression of proteins that trigger resistance to viral infection, bind simultaneously to two or more different cytokine receptors, a process called receptor hetero-oligomerization. Generally these cytokine receptors bind to and activate two members of the JAK kinase family. Nonetheless, the signaling pathways activated by all cytokine receptors are broadly similar (see Figure 16-6), and receptor dimerization induced by hormone binding is common to many other receptor types that activate tyrosine kinases.
Interleukin 2 (IL-2) is essential for the formation of functional T cells, an essential component of the immune system (see Chapter 23). The IL-2 receptor is an oligomer of three different subunits termed alpha, beta, and gamma. The gamma chain is also an essential subunit of the receptors for several other interleukins, including IL-4, IL-7, IL-9, IL-15, and IL-21, all cytokines that are essential for formation of the antibody-producing B cells and other types of immune-system cells. Severe combined immunodeficiency (SCID) is a genetic disease in which neither T nor B cells are produced. People with SCID cannot cope with any bacterial or viral infection, so they must be kept in a sterile environment (like the famous “bubble boy”). Many cases of SCID are due to a deficiency in the IL-2 receptor gamma chain. These children can now be cured by gene therapy: a viral vector (see Figure 6-30) is used to introduce a functional gamma chain gene into the hematopoietic stem cells that generate all immune-system cells (see Chapter 23).
Here we focus on the simpler case of receptor homodimerization. The interaction of one erythropoietin molecule with two identical erythropoietin receptor (EpoR) proteins, as depicted in Figure 16-8, exemplifies the binding of this group of cytokines to their receptors. The structure of the GH-GH receptor complex is similar, and detailed mutagenesis studies have shown that only eight amino acids in growth hormone (green) contribute 85 percent of the energy that is responsible for tight receptor binding; these amino acids are distant from one another in the primary sequence but adjacent in the folded protein (Figure 16-9). Because of the multiple weak, noncovalent forces (i.e., ionic, van der Waals, and hydrophobic interactions) involved in this binding as well as molecular complementarity between the interacting surfaces of the receptor and ligand, the binding is extremely tight; a dissociation constant (Kd) of about 10−10 M is characteristic of most cytokine receptors. Thus very low concentrations of GH and most other cytokines—about 1 microgram per liter (roughly one part in a billion)—are sufficient to activate cytokine receptors.
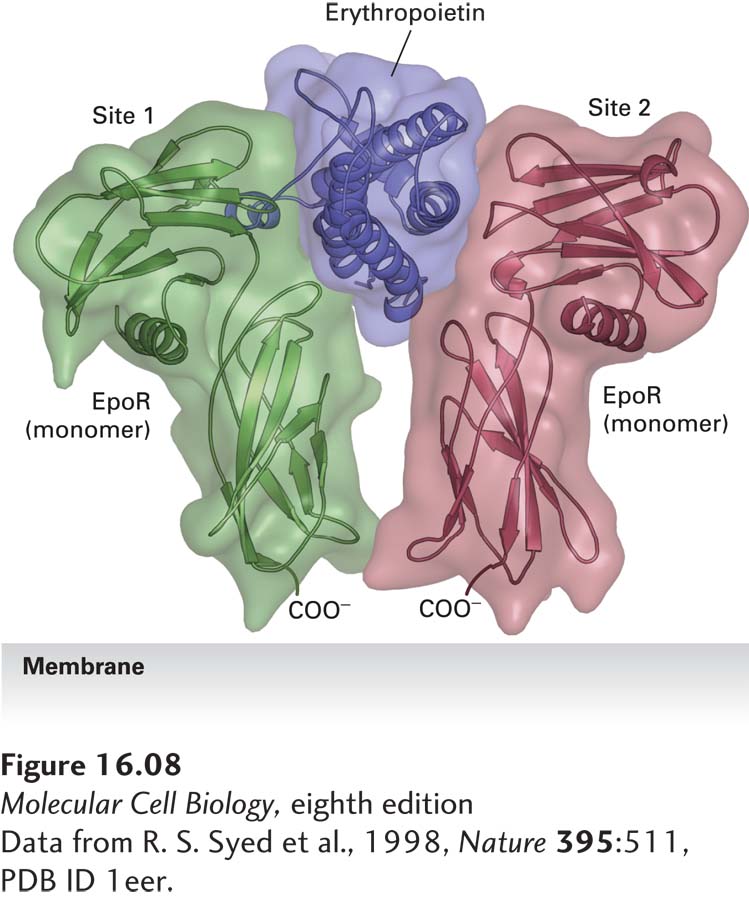
FIGURE 16-8 Structure of erythropoietin bound to an erythropoietin receptor. Like other cytokines, erythropoietin (Epo) contains four conserved long α helices that are folded in a particular arrangement. The activated erythropoietin receptor (EpoR) is a dimer of identical subunits; the extracellular domain of each monomer is constructed of two subdomains, each containing seven conserved β strands folded in a characteristic fashion. Side chains of residues on two of the α helices in Epo, termed site 1, contact loops on one EpoR monomer, while residues on the two other Epo α helices, termed site 2, bind to the same loop segments in a second receptor monomer, thereby stabilizing the dimeric receptor in a specific conformation.
[Data from R. S. Syed et al., 1998, Nature 395:511, PDB ID 1eer.]
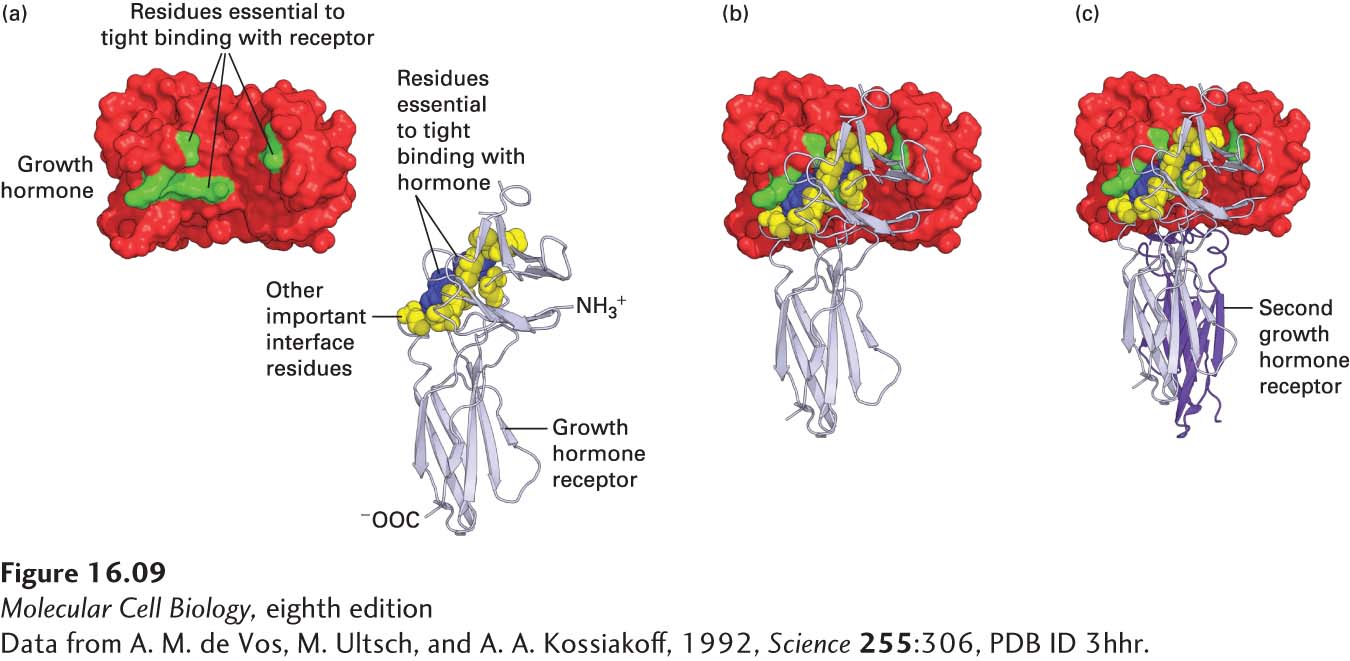
EXPERIMENTAL FIGURE 16-9 Growth hormone binds to its receptor through multiple weak, noncovalent forces. (a) As determined from the three-dimensional structure of the 1 growth hormone:2 growth hormone receptor complex, 28 amino acids in the hormone are at the binding interface with one receptor molecule. To determine which amino acids are important in ligand-receptor binding, researchers mutated each of these amino acids, one at a time, to alanine and measured the effect on receptor binding. From this study, it was found that only 8 amino acids on growth hormone (green) contribute 85 percent of the energy that is responsible for tight receptor binding; these amino acids are distant from one another in the primary sequence, but adjacent in the folded protein. Similar studies showed that two tryptophan residues (blue) in the receptor contribute most of the energy responsible for tight binding of growth hormone, although other amino acids at the interface with the hormone (yellow) are also important. (b) As with the Epo receptor, binding of growth hormone to one receptor molecule is followed by (c) binding of a second receptor (purple) to the opposite side of the hormone; this binding involves the same set of yellow and blue amino acids on the receptor, but different residues on the hormone. See B. Cunningham and J. Wells, 1993, J. Mol. Biol. 234:554, and T. Clackson and J. Wells, 1995, Science 267:383.
[Data from A. M. de Vos, M. Ultsch, and A. A. Kossiakoff, 1992, Science 255:306, PDB ID 3hhr.]
Cytokine receptors do not possess intrinsic enzyme activity. Rather, a JAK kinase is tightly bound to the cytosolic domain of the receptor (Figure 16-10). Each of the four members of the JAK family of kinases contains an N-terminal receptor-binding domain, a C-terminal kinase domain that is normally poorly active catalytically, and a middle “pseudokinase” domain that regulates kinase activity by an unknown mechanism. (JAKs are so named because when they were first cloned and characterized, their function was unknown; they were termed just another kinase.) These kinases become activated after ligand binding and receptor dimerization (Figure 16-10, step 1).
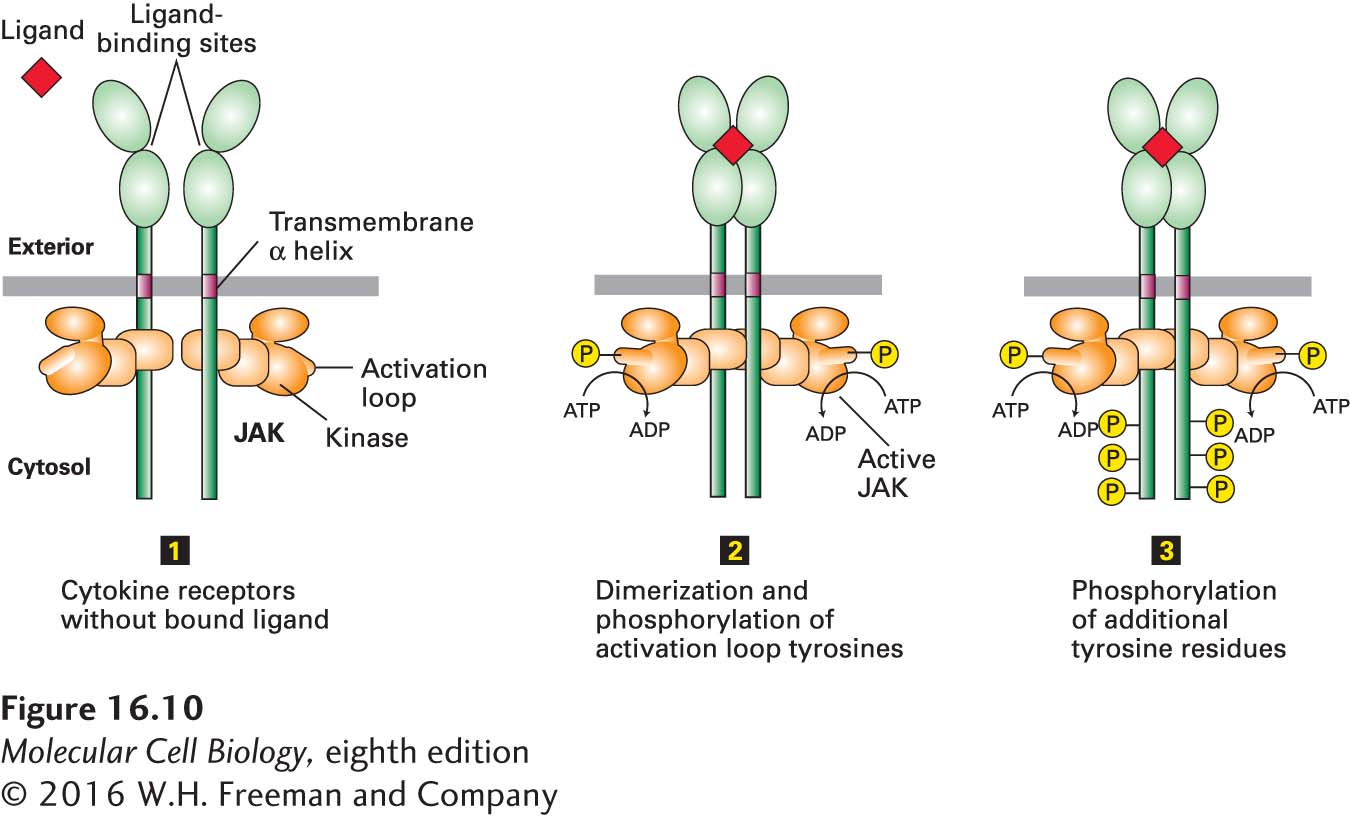
FIGURE 16-10 General structure and activation of cytokine receptors. The cytosolic domain of a cytokine receptor binds tightly and irreversibly to a JAK protein tyrosine kinase. In the absence of ligand (step 1), two receptors form a homodimer, but the JAK kinases are poorly active. Ligand binding causes a conformational change that brings together the JAK kinase domains, which then phosphorylate each other on a tyrosine residue in a region called the activation loop, activating the kinases (step 2). The active JAK kinases then phosphorylate multiple tyrosine residues in the receptor cytosolic domain (step 3). The resulting phosphotyrosines function as docking sites for signal-transducing proteins, including the STAT proteins.
Some cytokine receptors, such as the Epo receptor, are homodimers in the absence of ligand; others dimerize only in the presence of ligand. In both cases, ligand binding triggers a conformational change in the JAKs such that they can phosphorylate each other on a critical tyrosine in a region called the activation loop (Figure 16-10, step 2). As with many other kinases, phosphorylation of the activation loop leads to a conformational change in the kinase that enhances the affinity of the enzyme for ATP or the substrate to be phosphorylated, thereby increasing kinase activity (Figure 16-10, step 3). One piece of evidence for this activation mechanism comes from study of a mutant JAK2 in which the critical tyrosine is mutated to phenylalanine. The mutant JAK2 binds normally to EpoR, but cannot be phosphorylated and is catalytically inactive. In erythroid progenitor cells, expression of this mutant JAK2 in greater than normal amounts totally blocks EpoR signaling because the mutant JAK2 binds to the majority of EpoR proteins, preventing binding and functioning by the wild-type JAK2 protein. This type of mutation, referred to as a dominant-negative mutation, causes loss of function even in cells that carry copies of the wild-type gene because the mutant protein prevents the normal protein from functioning (see Chapter 6).


