Multiple Mechanisms Down-
In the last chapter, we saw several ways in which signaling from G protein–
732
Phosphotyrosine Phosphatases Phosphotyrosine phosphatases are dephosphorylating enzymes that specifically hydrolyze phosphotyrosine linkages on specific target proteins. An excellent example of how phosphotyrosine phosphatase enzymes function to suppress the activity of protein tyrosine kinases is provided by SHP1, a phosphatase that negatively regulates signaling by several types of cytokine receptors. Its role was first identified by analysis of mice lacking this protein, which died because of excess production of several types of blood cells, including erythrocytes.
SHP1 dampens cytokine signaling by binding to a cytokine receptor and inactivating the associated JAK protein, as depicted in Figure 16-13a. In addition to a phosphatase catalytic domain, SHP1 has two SH2 domains. When cells are in the resting state, unstimulated by a cytokine, one of the SH2 domains in SHP1 physically binds to and masks the catalytic site in the enzyme’s phosphatase domain. In the stimulated state, however, this blocking SH2 domain binds to a specific phosphotyrosine residue in the activated receptor. The conformational change that accompanies this binding unmasks the SHP1 catalytic site and also brings it adjacent to the phosphotyrosine residue in the activation loop of the JAK associated with the receptor. By removing this phosphate, SHP1 inactivates the JAK, so that it can no longer phosphorylate the receptor or other substrates (such as STATs) unless additional cytokine molecules bind to cell-
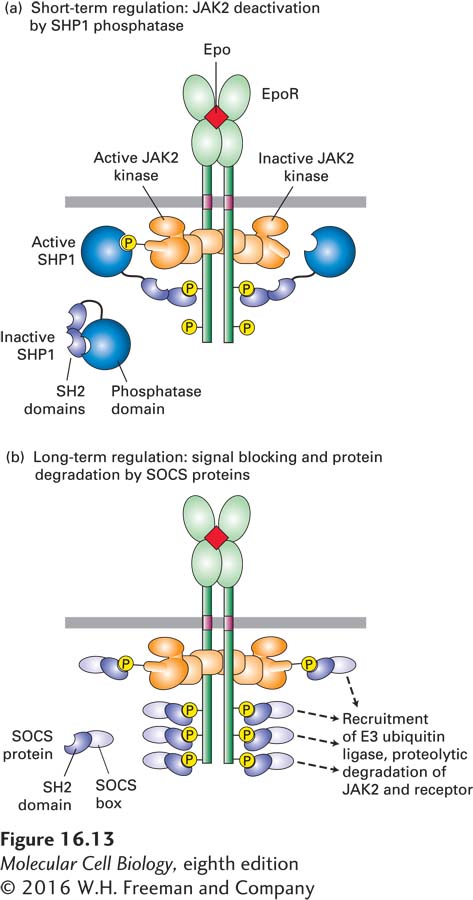
733
SOCS Proteins In a classic example of negative feedback, among the genes whose transcription is induced by STAT proteins are those encoding a class of small proteins termed suppressor of cytokine signaling (SOCS) proteins, which terminate signaling by cytokine receptors. All SOCS proteins contain an SH2 domain and another domain, called the SOCS box, that recruits components of E3 ubiquitin ligases (see Figure 3-31). The SOCS SH2 domain binds to specific phosphotyrosines on an activated receptor (Figure 16-13b); as a result, the receptor itself, as well as the associated JAK kinase, becomes polyubiquitinylated (a polymer of ubiquitins is covalently attached to the side chain of a lysine) and is then degraded in proteasomes (see Chapter 3), thereby permanently turning off all JAK2-
Studies with cultured mammalian cells have shown that the receptor for growth hormone, which belongs to the cytokine receptor family, is down-