Binding of Ligand Promotes Dimerization of an RTK and Leads to Activation of Its Intrinsic Tyrosine Kinase
All RTKs have three essential components: an extracellular domain containing a ligand-binding site, a single hydrophobic transmembrane α helix, and a cytosolic segment that includes a domain with protein tyrosine kinase activity (Figure 16-14). Most RTKs are monomeric, and ligand binding to the extracellular domain induces formation of receptor dimers. As with cytokine receptors, the formation of functional dimers is a necessary step in activation of all RTKs.
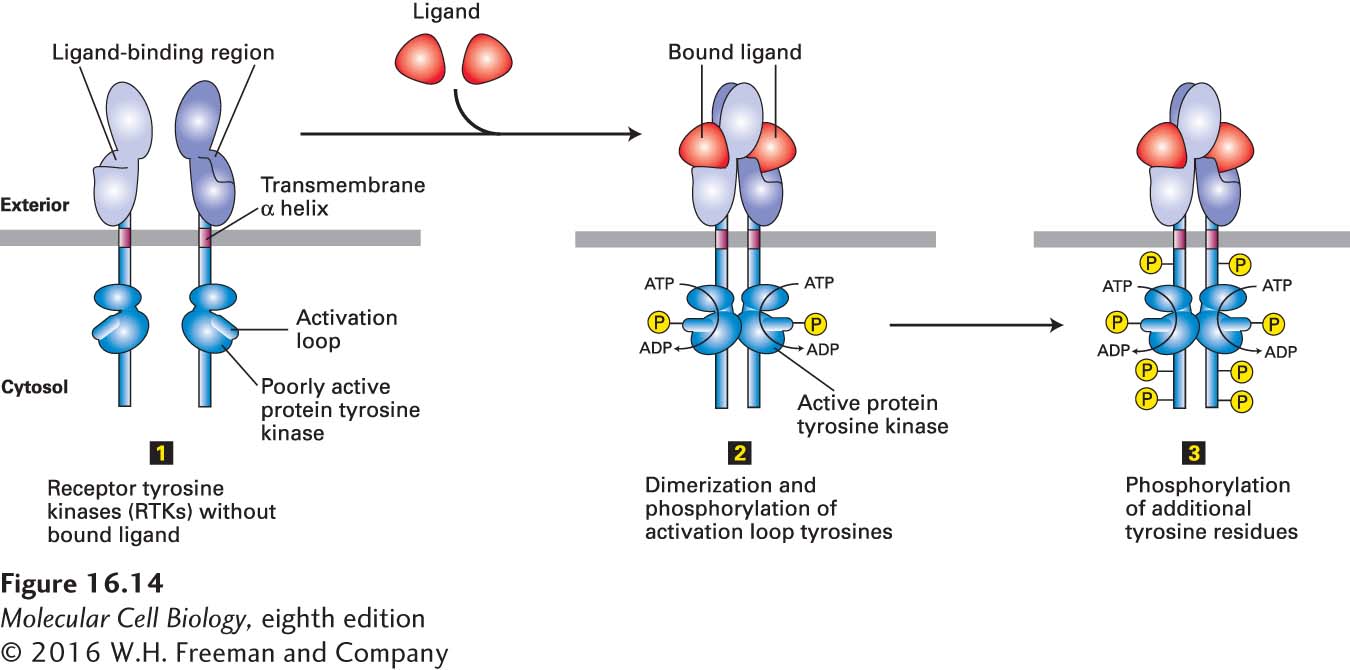
FIGURE 16-14 General structure and activation of receptor tyrosine kinases (RTKs). The cytosolic domain of RTKs contains an intrinsic protein tyrosine kinase catalytic site. In the absence of ligand (step 1), RTKs generally exist as monomers with poorly active kinases. Binding of two ligands to the extracellular domains of two RTKs forms or stabilizes an activated dimeric receptor. This brings together two poorly active kinases such that each one phosphorylates the other on a tyrosine residue in the activation loop (step 2). Phosphorylation causes the loop to move out of the kinase catalytic site, thus increasing the ability of ATP and/or the protein substrate to bind. The activated kinase then phosphorylates several tyrosine residues in the receptor’s cytosolic domain (step 3). The resulting phosphotyrosines function as docking sites for SH2 and other binding domains on downstream signal-transducing proteins.
RTK activation can be summarized as follows: In the resting, unstimulated (no ligand bound) state, the intrinsic kinase activity of an RTK is very low (Figure 16-14, step 1). Like most other kinases, including the JAK kinases discussed earlier, RTKs contain a flexible domain termed the activation loop. In the resting state, the activation loop is nonphosphorylated and assumes a conformation that blocks kinase activity. In some receptors (e.g., the insulin receptor), it prevents binding of ATP. In others (e.g., the FGF receptor), it prevents binding of substrate. Binding of ligand causes a conformational change that promotes dimerization of the extracellular domains of RTKs, which brings their transmembrane segments—and therefore their cytosolic domains—close together. In a manner similar to the activation of the JAK kinases, the kinase in each subunit then phosphorylates a particular tyrosine residue in the activation loop of the other subunit (Figure 16-14, step 2). This phosphorylation leads to a conformational change in the activation loop that unblocks kinase activity by reducing the KD for ATP or the substrate to be phosphorylated. The resulting enhanced kinase activity can then phosphorylate additional tyrosine residues in the cytosolic domain of the receptor (Figure 16-14, step 3), which bind SH2 or other domains of signaling proteins, as well as phosphorylating other target proteins, leading to intracellular signaling.
Although dimerization is a necessary step in the activation of all RTKs, functional dimers can be formed in multiple ways. Many receptors are dimerized in a manner similar to the fibroblast growth factor (FGF) receptor (Figure 16-15), in which each of two FGF molecules binds simultaneously to the extracellular domains of two receptor subunits, stabilizing the dimer. FGF also binds tightly to heparan sulfate, a negatively charged polysaccharide component of some cell-surface proteins and of the extracellular matrix (see Chapter 20); this association enhances ligand binding and formation of a dimeric receptor-ligand complex (see Figure 16-15). The participation of the heparan sulfate is essential for efficient receptor activation.
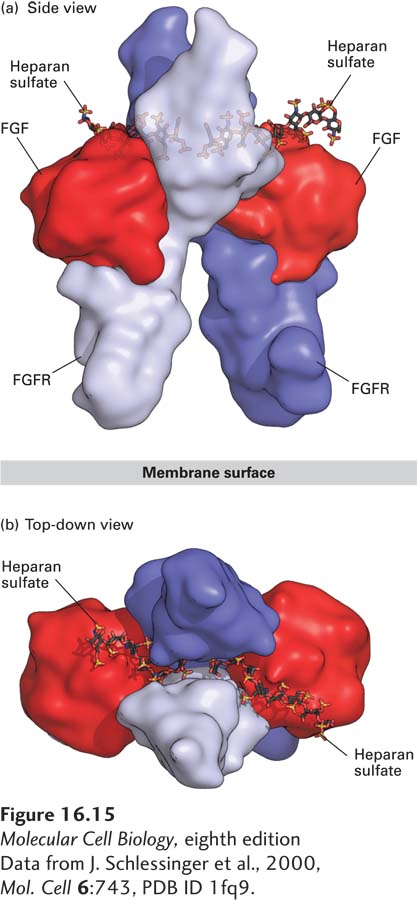
FIGURE 16-15 Structure of the extracellular domains of the active dimeric fibroblast growth factor (FGF) receptor, stabilized by binding of two ligands and by heparan sulfate. Shown here are side and top-down views of the complex comprising the extracellular domains of two FGF receptor (FGFR) monomers (purple and violet), two bound FGF molecules (red), and two short heparan sulfate chains, which bind tightly to FGF. (a) In the side view, the upper domain of one receptor monomer (purple) is seen situated behind that of the other (violet); the plane of the plasma membrane is at the bottom. A small segment of the extracellular domain whose structure is not known connects to the membrane-spanning α-helical segment of each of the two receptor monomers (not shown) that protrude downward into the membrane. (b) In the top view, the heparan sulfate chains are seen threading between and making numerous contacts with the upper domains of both receptor monomers. These interactions promote binding of the ligand to the receptor and receptor dimerization.
[Data from J. Schlessinger et al., 2000, Mol. Cell 6:743, PDB ID 1fq9.]
Yet other RTKs, such as the insulin receptor, form disulfide-linked dimers even in the absence of hormone; binding of ligand to this type of RTK alters its conformation in such a way that the receptor kinase becomes activated. This last example highlights the fact that simply having two receptor monomers in close contact is not sufficient for receptor activation—the proper conformational changes must accompany receptor dimerization to lead to tyrosine kinase activation. Once an RTK is locked into a functional dimeric state, its associated tyrosine kinase becomes activated.

