Homo- and Hetero-oligomers of Epidermal Growth Factor Receptors Bind Members of the Epidermal Growth Factor Family
Four RTKs participate in signaling by the many members of the epidermal growth factor (EGF) family of signaling molecules; these receptors are termed Erb-B1, 2, 3, and 4. In humans these receptors are also called HER (human epidermal growth factor receptor) 1, 2, 3, and 4, respectively. Epidermal growth factors and their receptors have been studied intensively because of their involvement in many human diseases; drugs targeting receptors that are overproduced in tumors or have activating mutations are used to treat many cancers, as we learn in Chapter 24.
In the resting state, the majority of EGF receptor molecules are monomeric. HER1 (Erb-B1) directly binds EGF as well as six other members of the EGF family: heparin-binding EGF (HB-EGF), transforming growth factor α (TGF-α), amphiregulin (AREG), epiregulin (EREG), epigen (EPGN), and betacellulin (BTC). Binding of any of these ligands leads to homodimerization of the HER1 extracellular domain (Figure 16-16). In the resting, monomeric, state, the β-hairpin in the segment of receptor domain II, termed the dimerization arm, forms a tight intramolecular interaction with domain IV. Binding of EGF induces a dramatic conformational change to the extracellular domain of HER1 in which the receptor “clamps down” on the hormone such that the dimerization arm becomes exposed. The dimerization arms from two activated receptors then bind tightly together, forming a stable receptor homodimer. In contrast to the FGF receptors (see Figure 16-15), the ligand does not directly stabilize the active homodimer.
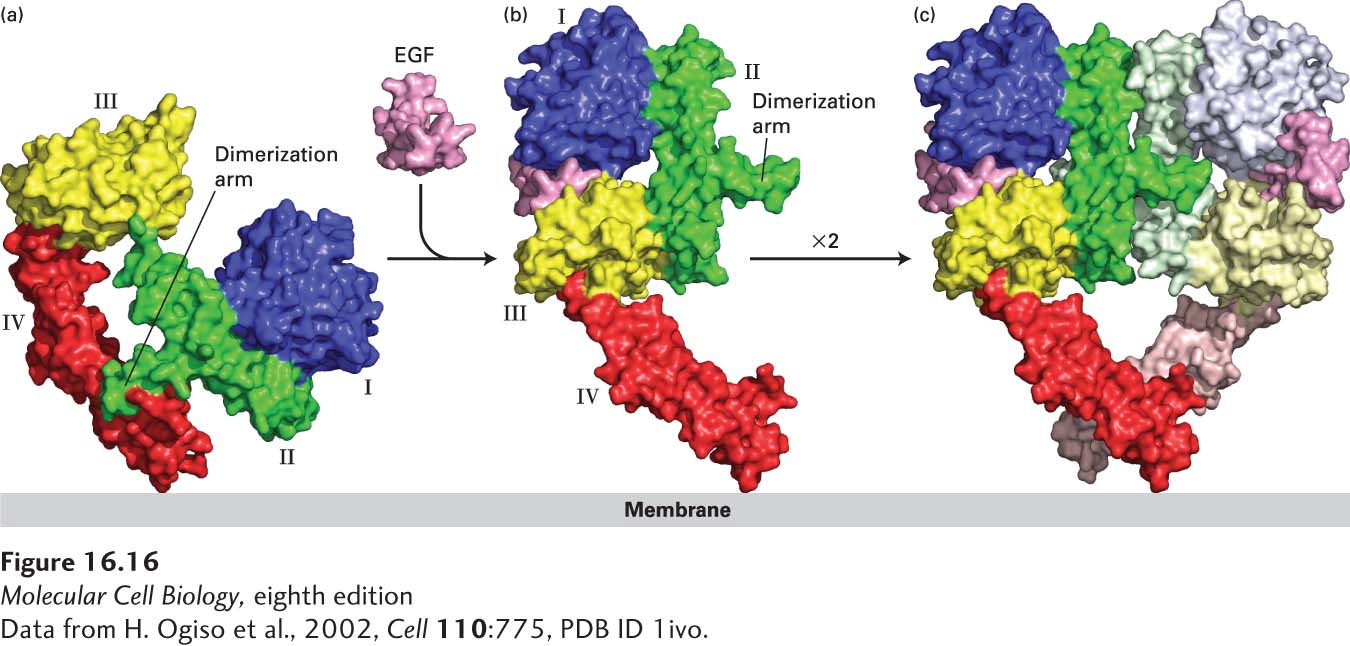
FIGURE 16-16 Ligand-induced dimerization of HER1, a human receptor for epidermal growth factor (EGF). (a) The extracellular region of all EGF receptors contains four domains: domains I (blue) and III (yellow) are closely related in sequence, as are domains II (green) and IV (red). In the absence of bound EGF, the receptor is mostly monomeric and the intracellular kinase is inactive. The extracellular region adopts a configuration in which the β-hairpin from domain II that forms the “dimerization arm” binds to domain IV of the same receptor molecule. (b) EGF binds simultaneously to domains I and III; binding induces a major conformational change in the extracellular domain such that the dimerization arm of domain II is now exposed. (c) Dimerization of two identical ligand-bound receptor monomers in the plane of the membrane occurs primarily through interactions between the dimerization arms of the two receptors.
[Data from H. Ogiso et al., 2002, Cell 110:775, PDB ID 1ivo.]
HER3 and HER4 also bind members of the EGF family. Neuregulins 1 and 2 (NRG1 and NRG2) bind to both HER3 and HER4; HB-EGF, NRG3, and NRG4 bind only to HER4. HER4, like HER1, can form stable homodimers after binding a growth factor, but HER3 cannot. So how can HER3 signal? The answer involves HER2, which does not bind a ligand. Rather, HER2 exists on the plasma membrane in a conformation that is very similar to that of HER1 with a bound EGF; the HER2 dimerization arm protrudes outward and is able to bind to and form heterodimers with ligand-bound HER3 as well as with ligand-bound HER1 and HER4 (Figure 16-17). HER2 can signal only by forming heterocomplexes with ligand-bound HER1, HER3, or HER4; thus it facilitates signaling by all EGF family members (see Figure 16-17). Even though HER3 lacks a functional kinase domain, it can still participate in signaling; after binding a ligand, it heterodimerizes with HER2 and becomes phosphorylated by the HER2 kinase, activating downstream signal transduction pathways.
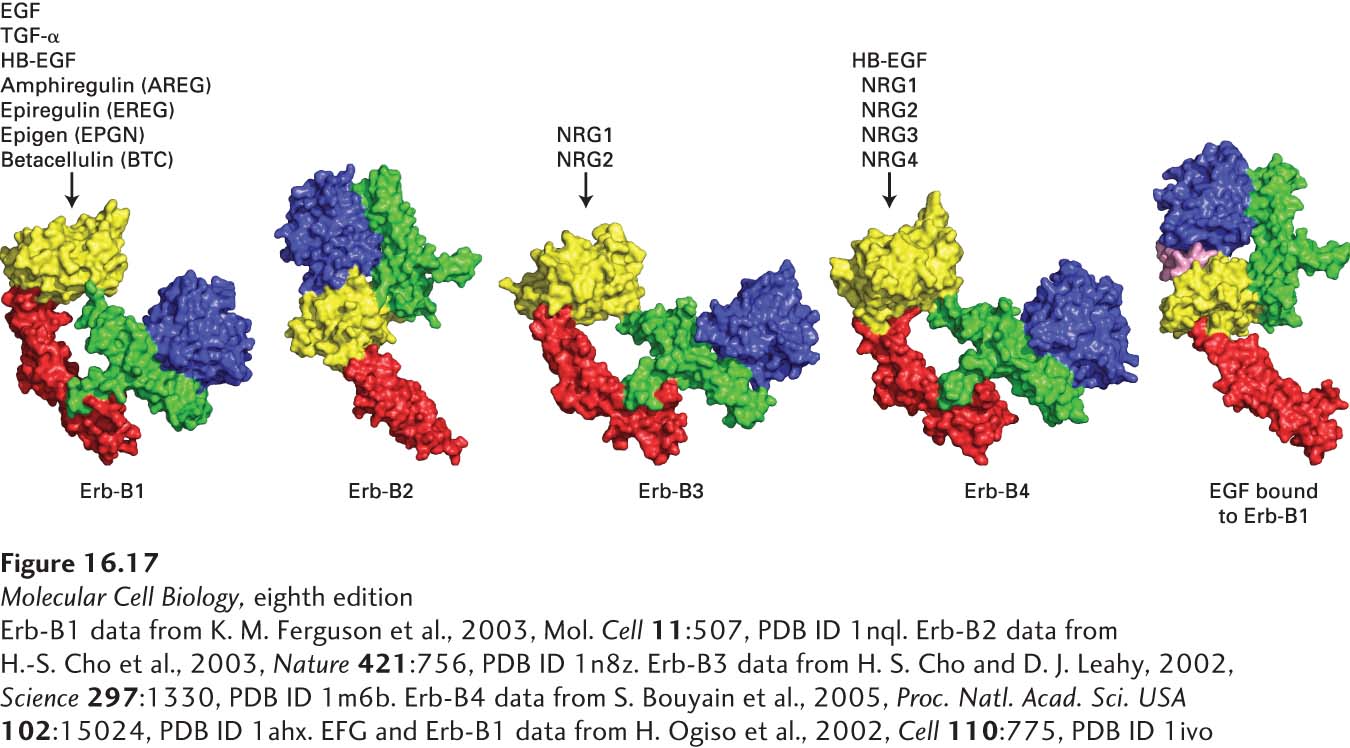
FIGURE 16-17 The HER family of receptors and their ligands. Humans and mice express four receptor tyrosine kinases, denoted HER1, 2, 3, and 4 in humans and Erb-B1, 2, 3, and 4 in mice and other animals. Only the extracellular domains of these receptors are depicted here. These receptors bind epidermal growth factor (EGF) and the other EGF family members: heparin-binding EGF (HB-EGF), transforming growth factor α (TGF-α), amphiregulin (AREG), epiregulin (EREG), epigen (EPGN), betacellulin (BTC), and the four neuregulins. Note that Erb-B2 (HER2), which does not directly bind a ligand, exists in a conformation that is very similar to that of the activated Erb-B1 with a bound EGF. Erb-B2 can form a heterodimer with ligand-activated Erb-B1, 3, or 4; thus Erb-B2 facilitates signaling by all EGF family members. Erb-B3 (HER3) has a very poorly active kinase domain and can signal only when complexed with Erb-B2.
[Erb-B1 data from K. M. Ferguson et al., 2003, Mol. Cell 11:507, PDB ID 1nql. Erb-B2 data from H.-S. Cho et al., 2003, Nature 421:756, PDB ID 1n8z. Erb-B3 data from H. S. Cho and D. J. Leahy, 2002, Science 297:1330, PDB ID 1m6b. Erb-B4 data from S. Bouyain et al., 2005, Proc. Natl. Acad. Sci. USA 102:15024, PDB ID 1ahx. EFG and Erb-B1 data from H. Ogiso et al., 2002, Cell 110:775, PDB ID 1ivo.]
Thus an increase in HER2 on the cell surface makes a cell more sensitive to signaling by many EGF family members because the rate at which the signaling heterodimers are formed after ligand binding will be enhanced. As we learn in Chapter 24, understanding the HERs has helped explain why a common form of breast cancer, in which the HER2 gene is amplified and HER2 is overexpressed, is so dangerous and has led to important drug therapies. Overexpression of HER2 makes the tumor cells sensitive to growth stimulation by low levels of any member of the EGF family of growth factors—levels that would not stimulate proliferation of cells with normal HER2 levels.

