Activation of the EGF Receptor Results in the Formation of an Asymmetric Active Kinase Dimer
As is the case with most receptor tyrosine kinases, the kinase domain in the FGF receptor is activated by phosphorylation of a tyrosine in the activation loop. In contrast, activation of the EGF receptor kinases does not involve loop phosphorylation; their mechanism of activation was uncovered only recently through structural studies of the receptor cytosolic domain in both the active and inactive states. The kinase domains are separated from the transmembrane segment by a so-called juxtamembrane segment, colored blue in Figure 16-18. In the inactive, monomeric state, the activation loop is localized to the active site of the kinase, blocking its activity; in this way the kinase is maintained in the “off” state (Figure 16-18, left). Receptor dimerization generates an asymmetric kinase dimer (Figure 16-18, right) in which one kinase domain—termed the donor—binds the second kinase domain—the acceptor. This binding changes the conformation of the top lobe of the receiver, causing the activation loop to move out of the kinase active site and activating the kinase.
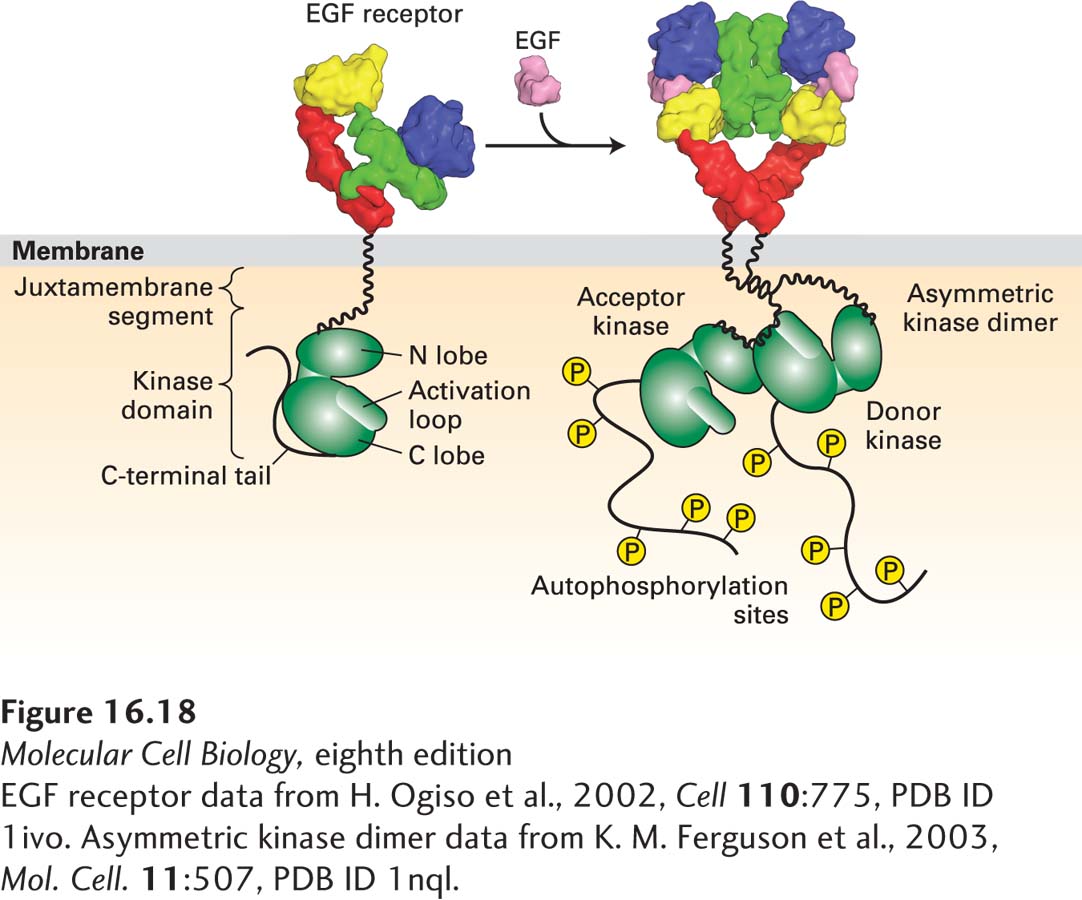
FIGURE 16-18 Activation of the EGF receptor by EGF results in the formation of an asymmetric kinase domain dimer. In the inactive, monomeric state, the activation loop is localized to the kinase active site and thus inhibits kinase activation. Receptor dimerization generates an asymmetric kinase dimer such that the C-terminal C-lobe of the donor kinase binds to the N-terminal N-lobe of the acceptor kinase in the opposite receptor; the dimer is stabilized by interactions between the juxtamembrane segments of the two receptors. These interactions cause a conformational change that removes the activation loop from the kinase site of the acceptor kinase, activating its kinase activity. The active kinase then phosphorylates tyrosine residues in the C-terminal segments of the receptor cytosolic domain.
[EGF receptor data from H. Ogiso et al., 2002, Cell 110:775, PDB ID 1ivo. Asymmetric kinase dimer data from K. M. Ferguson et al., 2003, Mol. Cell. 11:507, PDB ID 1nql.]
Thus evolution has produced many variations on the theme of this simple ligand-RTK mechanism: RTKs are activated by dimerization, but different receptors use different mechanisms to accomplish this. Similarly, kinases become activated by movement of the activation loop away from the kinase catalytic site, but different receptors use different mechanisms to accomplish this task. Down-regulation of signaling from RTKs is also common, and different mechanisms have evolved to accomplish this task as well.
