Receptor Tyrosine Kinases Are Linked to Ras by Adapter Proteins
In order for activated RTKs and cytokine receptors to activate Ras, two cytosolic proteins—GRB2 and Sos—must first be recruited to provide a link between the receptor and Ras (Figure 16-21). GRB2 is an adapter protein, meaning that it has no enzyme activity and serves as a link, or scaffold, between two other proteins—in this case, between the activated receptor and Sos. Sos is a guanine nucleotide exchange protein (GEF) that catalyzes conversion of inactive GDP-bound Ras to the active GTP-bound form.
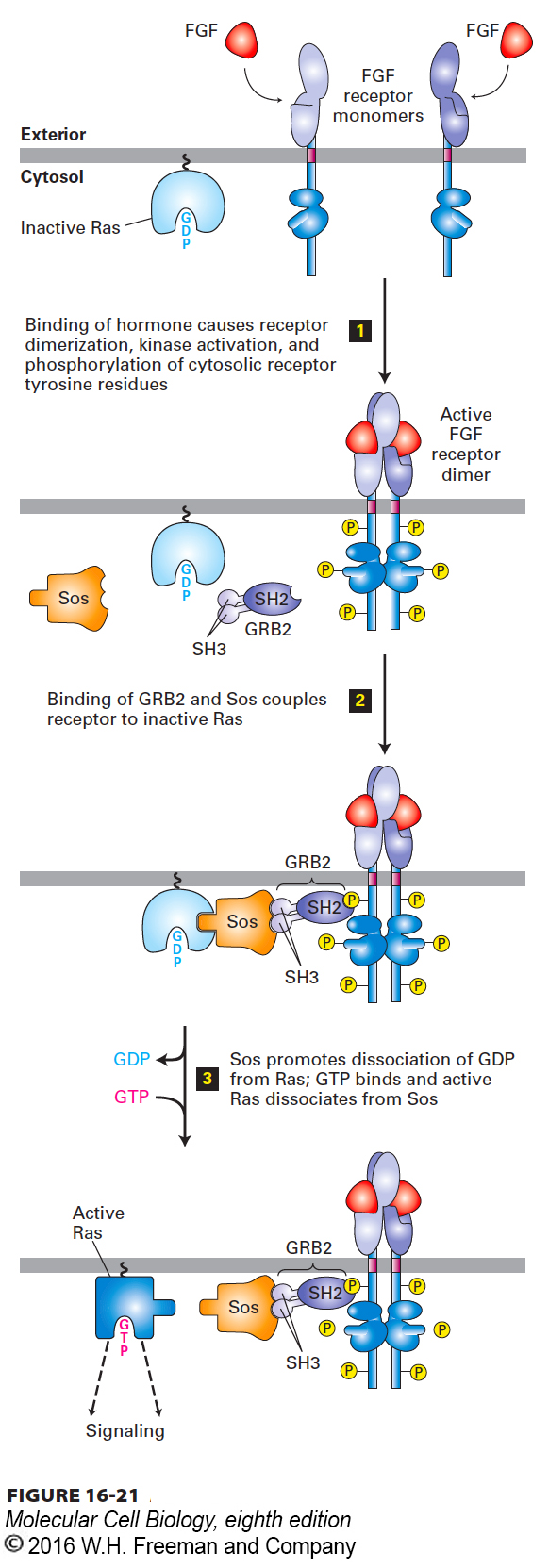
FIGURE 16-21 Activation of Ras following ligand binding to receptor tyrosine kinases (RTKs) or cytokine receptors. The receptors for fibroblast growth factor (FGF) and many other growth factors are RTKs. The SH2 domain of the cytosolic adapter protein GRB2 binds to a specific phosphotyrosine on an activated, ligand-bound receptor, and the SH3 domains bind to the cytosolic Sos protein, bringing it near the cytosolic surface of the plasma membrane and close to its substrate, the inactive Ras·GDP. The guanine nucleotide exchange factor (GEF) activity of Sos then promotes formation of active Ras·GTP. Note that Ras is tethered to the cytosolic surface of the plasma membrane by a hydrophobic farnesyl anchor (see Figure 7-19). See J. Schlessinger, 2000, Cell 103:211, and M. A. Simon, 2000, Cell 103:13.
GRB2 is able to serve as an adapter protein because of its SH2 domain, which binds to a specific phosphotyrosine residue in the cytosolic domain of an activated RTK (or cytokine receptor). In addition to its SH2 domain, the GRB2 adapter protein contains two SH3 domains, which bind to Sos (see Figure 16-21). Like phosphotyrosine-binding SH2 domains, SH3 domains are present in a large number of proteins involved in intracellular signaling. Although the three-dimensional structures of various SH3 domains are similar, their specific amino acid sequences differ. The SH3 domains in GRB2 selectively bind to proline-rich sequences in Sos; different SH3 domains in other proteins bind to proline-rich sequences distinct from those in Sos.
Proline residues play two roles in the interaction between an SH3 domain in an adapter protein (e.g., GRB2) and a proline-rich sequence in another protein (e.g., Sos). First, the proline-rich sequence assumes an extended conformation that permits extensive contacts with the SH3 domain, thereby facilitating interaction. Second, a subset of the prolines fit into binding “pockets” on the surface of the SH3 domain (Figure 16-22). Several nonproline residues also interact with the SH3 domain and are responsible for determining the binding specificity. Hence the binding of proteins to SH3 and to SH2 domains follows a similar strategy: certain residues provide the key structural motif necessary for binding, and neighboring residues confer specificity to the binding.
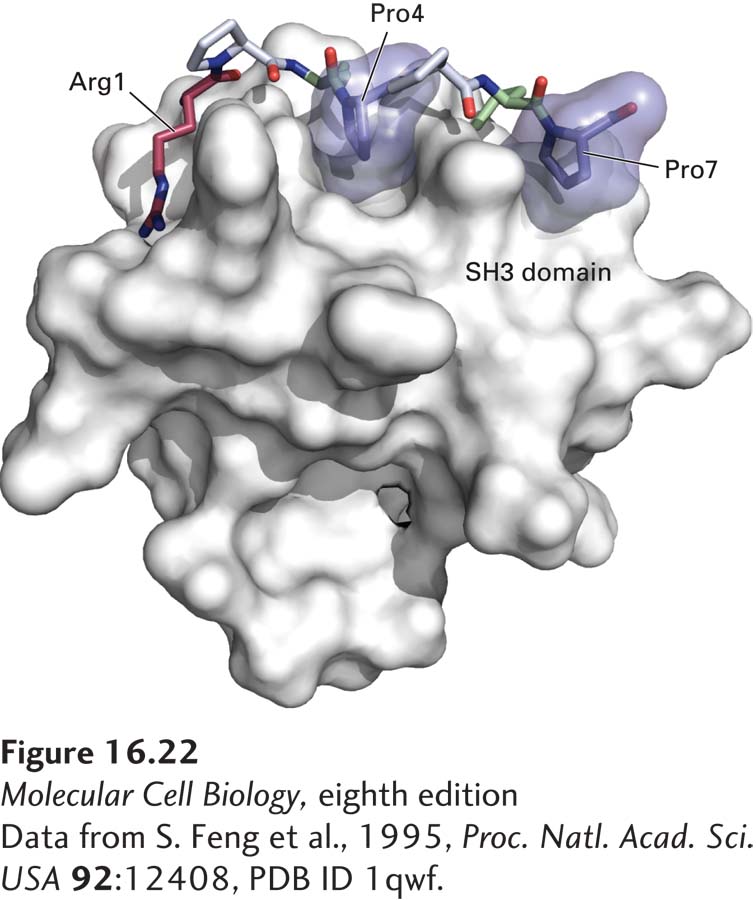
FIGURE 16-22 Surface model of an SH3 domain bound to a target peptide. The short, proline-rich target peptide is shown as a space-filling model. In this target peptide, two prolines (Pro4 and Pro7, dark blue) fit into binding pockets on the surface of the SH3 domain. Interactions involving an arginine (Arg1, red), two other prolines (gray), and other residues in the target peptide (green) determine the specificity of binding.
[Data from S. Feng et al., 1995, Proc. Natl. Acad. Sci. USA 92:12408, PDB ID 1qwf.]

