Biochemical and x-ray crystallographic studies have provided a detailed picture of how phosphorylation activates MAP kinase. As in JAK kinases and receptor tyrosine kinases, the catalytic site in the inactive, nonphosphorylated form of MAP kinase is blocked by an activation loop (Figure 16-25a). Binding of MEK to MAP kinase destabilizes the loop structure, resulting in exposure of tyrosine 185, which is buried in the inactive conformation. Following phosphorylation of this critical tyrosine, MEK phosphorylates the neighboring threonine 183 (Figure 16-25b). Both the phosphorylated tyrosine and the phosphorylated threonine residue in MAP kinase interact with additional amino acids, thereby conferring an altered conformation on the loop region, which in turn permits binding of ATP to the catalytic site, which, as in all kinases, is in the groove between the upper and lower kinase domains. The phosphotyrosine residue (pY185) also plays a key role in binding specific substrate proteins to the surface of MAP kinase. Phosphorylation promotes not only the catalytic activity of MAP kinase, but also its dimerization. The dimeric form of MAP kinase is translocated to the nucleus, where it regulates the activity of many nuclear transcription factors.
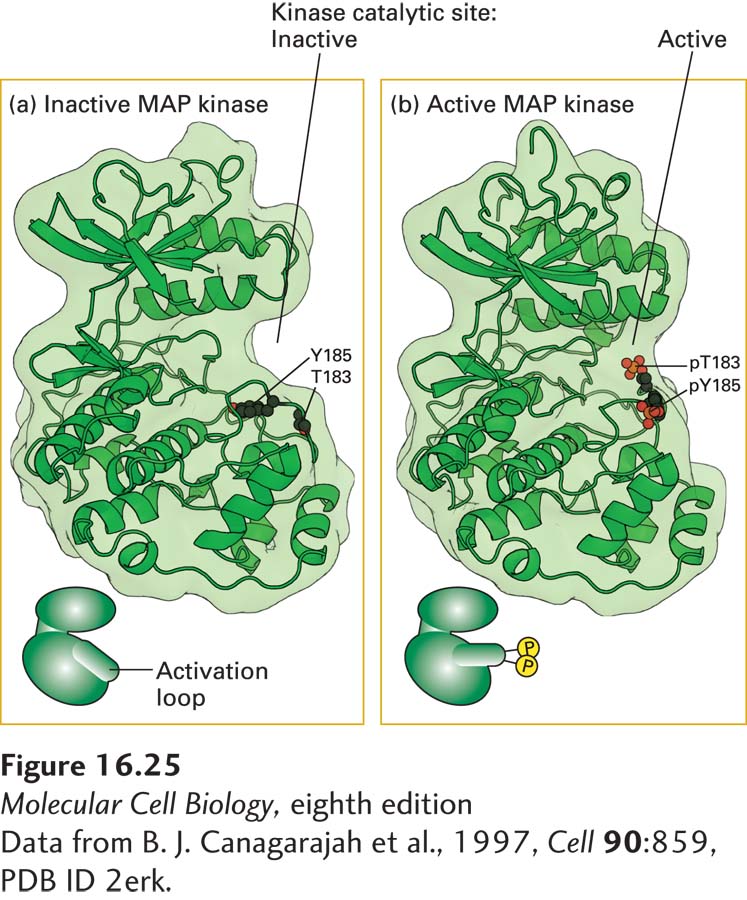
FIGURE 16-25 Structures of inactive, nonphosphorylated MAP kinase and the active, phosphorylated form. (a) In inactive MAP kinase, the activation loop is in a conformation that blocks the kinase active site. (b) Phosphorylation by MEK at tyrosine 185 (Y-185) and threonine 183 (T-183) leads to a marked conformational change in the activation loop. This activating change promotes both binding of its substrates—ATP and its target proteins—to MAP kinase and its dimerization. A similar phosphorylation-dependent mechanism activates JAK kinases and the intrinsic kinase activity of RTKs.
[Data from B. J. Canagarajah et al., 1997, Cell 90:859, PDB ID 2erk.]

