TGF-β is synthesized with a long N-terminal prodomain that is cleaved off in the Golgi complex. The monomeric form of TGF-β contains three conserved intramolecular disulfide linkages. An additional cysteine in the center of each monomer links TGF-β monomers into functional homodimers and heterodimers (see Figure 16-3a), which are formed in the endoplasmic reticulum. The prodomain remains noncovalently attached to the TGF-β growth-factor domain as the protein is secreted and prevents binding of TGF-β to its cell-surface receptors. The latent prohormone–TGF-β complex is stored near the secreting cell, attached to specific components of the extracellular matrix.
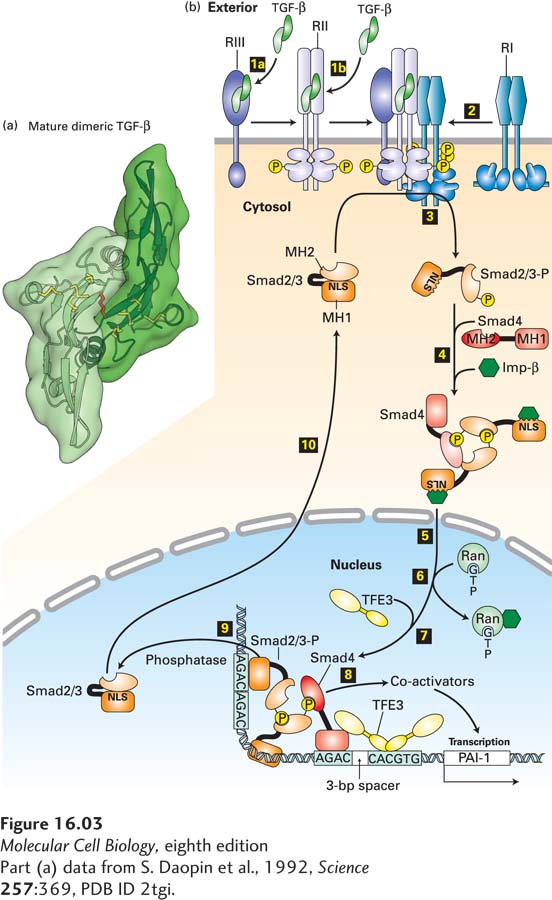
FIGURE 16-3 TGF-β/Smad signaling pathway. (a) Ribbon diagram structure of a mature TGF-β dimer. The three intrachain disulfide linkages (yellow) in each monomer form a cystine-knot domain; another disulfide bond (red) links the two monomers. (b) Step 1a: In some cells, TGF-β binds to the type III TGF-β receptor (RIII), which presents TGF-β to the type II receptor (RII). Step 1b: In other cells, TGF-β binds directly to RII, a constitutively active kinase. Step 2: Ligand-bound RII recruits and phosphorylates the juxtamembrane segment of the type I TGF-β receptor (RI), which does not directly bind TGF-β. This releases the inhibition of RI kinase activity. Step 3: Activated RI then phosphorylates Smad2 or Smad3 (shown here as Smad2/3), causing a conformational change that unmasks its nuclear-localization signal (NLS). Step 4: Two phosphorylated molecules of Smad2/3 bind to a co-Smad (Smad4) molecule, which is not phosphorylated, and to an importin, forming a large cytosolic complex. Steps 5 and 6: After the entire complex translocates into the nucleus, Ran·GTP causes dissociation of the importin, as discussed in Chapter 13. Step 7: A nuclear transcription factor (e.g., TFE3) then associates with the Smad2/3/Smad4 complex, forming an activation complex that cooperatively binds to regulatory sequences of a target gene. Step 8: This complex then recruits transcriptional co-activators and induces gene transcription (see Chapter 9). Smad2/3 is dephosphorylated by a nuclear phosphatase (step 9) and recycles through a nuclear pore to the cytosol (step 10), where it can be reactivated by another TGF-β receptor complex. Shown at the bottom is the activation complex for the gene encoding plasminogen activator inhibitor (PAI-1); similar transcription complexes activate expression of genes encoding other extracellular-matrix proteins such as fibronectin. See A. Moustakas and C.-H. Heldin, 2009, Development 136:3699, and D. Clarke and X. Liu, 2008, Trends Cell Biol. 18:430.
[Part (a) data from S. Daopin et al., 1992, Science 257:369, PDB ID 2tgi.]
Several mechanisms, including protease digestion, release the active TGF-β from the extracellular matrix and lead to quick activation of cell signaling—an important feature of many signaling pathways. A second important and unusual way of activating TGF-β emerged from the recognition that the prodomains of TGF-β1 and TGF-β3 contain a three-amino-acid motif, RGD (Arg-Gly-Asp), that binds to a class of cell-surface membrane proteins termed integrins (discussed in Chapter 20); integrins bind to RGD motifs in many extracellular-matrix proteins. Remarkably, binding of either of two integrins, termed αvβ6 or αvβ8, to the RGD motif in the TGF-β prodomain, followed by contraction of the integrin-expressing cell, physically pulls the prodomain away from the latent TGF-β, freeing it to bind to cell-surface receptors on the same cell and initiating signaling.

