Hedgehog Signaling in Vertebrates Requires Primary Cilia
The Hedgehog signaling pathway in vertebrates shares many conserved features with the Drosophila pathway, but there are also some striking differences. First, mammalian genomes contain three Hh genes and two Ptc genes, which are expressed differentially among various tissues. Second, mammals express three Gli transcription factors, which collectively perform the roles of the single Ci transcription factor in Drosophila. All other components of the Hh pathway in Drosophila also are conserved in mammals.
The most fascinating aspect of the mammalian Hh pathway is the importance of primary cilia. Cilia are long, plasma membrane–enveloped structures that protrude from the cell surface. The roles of cilia and flagella in specialized cell types—in tracheal cells in moving materials along the airway surface and in sperm in flagellum-powered locomotion—are well known (see Figure 1-14). Most vertebrate cells have a single cilium called the primary cilium (Figure 16-34), a slim, nonmotile structure that projects from the surface of nearly all vertebrate cells but is conspicuously absent in all invertebrate cell types that have been examined.
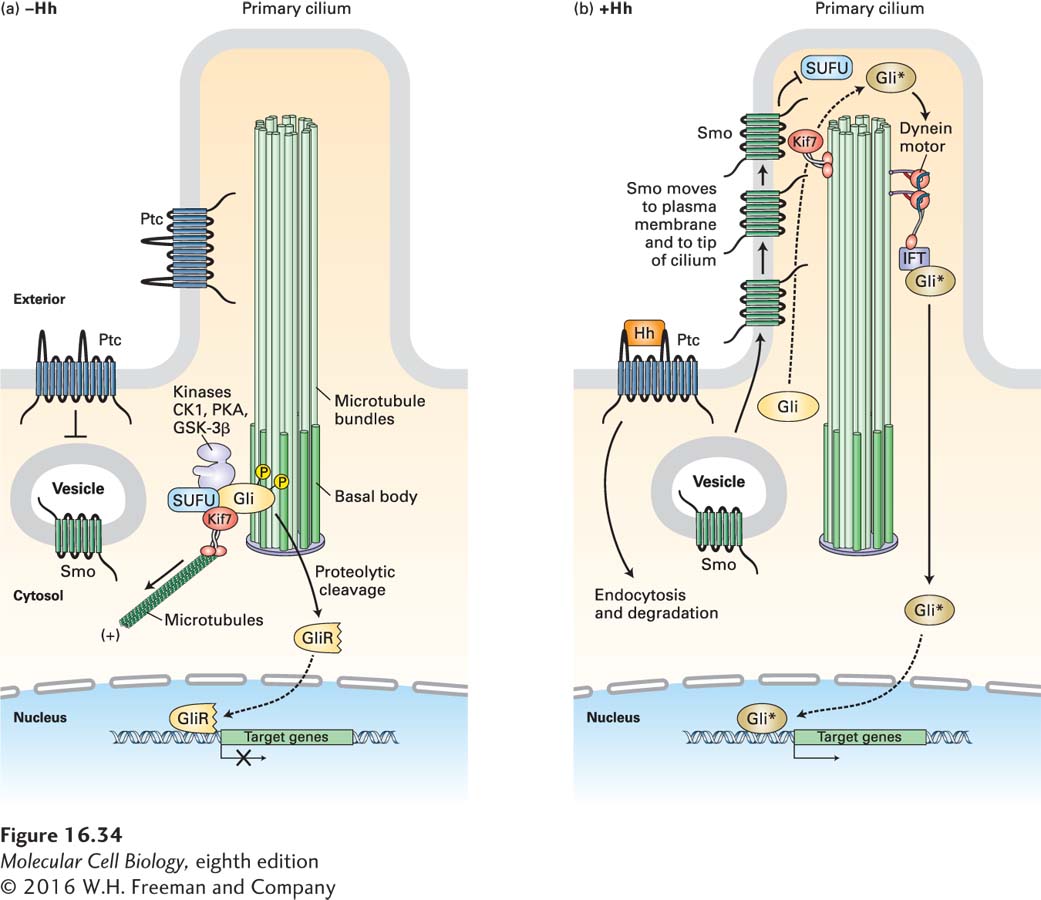
FIGURE 16-34 Hedgehog signaling in vertebrates. Hedgehog (Hh) signaling occurs in primary cilia, but otherwise the overall process is similar to that in Drosophila. (a) In the absence of Hh, Ptc is localized to the ciliary membrane and the base of the cilium. In an unknown manner, Ptc blocks the entry of Smo to the plasma membrane; Smo is present mainly in the membrane of internal vesicles. The kinesin Kif7 (the Cos2 homolog) binds to microtubules at the cilium base, where it prevents the transcription factor Gli (the vertebrate homolog of Ci) from entering the cilium. Kif7 and Gli are part of a complex that includes SUFU and the kinases CK1, PKA, and GSK3β, which phosphorylate Gli and promote its proteolytic cleavage to form the repressor GliR. (b) Hh binding triggers endocytosis and degradation of the Hh/Ptc complex, movement of Smo to the plasma membrane, and then, together with several proteins bound to it, its movement to the tip of the cilium. There Smo triggers dissociation of the SUFU-Gli complex. Rather than being degraded, Gli accumulates, becomes modified by addition of several phosphate and acetyl groups, forming the active Gli*, and is then transported down the cilium by a dynein motor protein. Gli* is then released into the cytosol, translocates into the nucleus, and activates gene expression. See J. Briscoe and P. Thérond, 2013, Nat. Rev. Mol. Cell Biol. 14:416.
As we will learn in Chapter 18, a cilium is extended and maintained by the transport of proteins and particles along a bundle of microtubules in its center; different intraflagellar transport (IFT) motor proteins move proteins and particles from the base of the cilium to the tip and in the opposite direction. Some of the first evidence for a role of cilia in Hh signaling came from a screen for mutations that altered early mouse development in a manner similar to that seen in embryos with altered Hh signaling: the mutant phenotypes included losses of certain types of cells in the neural tube that require high concentrations of one Hh protein to develop. Many of these mutations were in genes encoding IFT proteins, indicating a role for cilia (or flagella) in Hh signaling.
Subsequent analysis showed that, in the absence of Hh signaling, Ptc is localized to the membrane of the primary cilium and Smo is located in internal vesicles near the base of the cilium (Figure 16-34a). As in Drosophila, a cytosolic complex of Gli, SUFU, and several kinases leads to phosphorylation of Gli, its proteolytic cleavage, and translocation of a Gli fragment termed GliR into the nucleus, where it binds to regulatory regions of Gli-responsive genes and blocks their induction.
After Hh addition, Smo moves to the ciliary membrane and then to the tip of the primary cilium, while the Hh/Ptc complex is internalized and degraded (Figure 16-34b). These movements of Smo involve phosphorylation of the Smo C-terminal cytosolic domain by β-adrenergic receptor kinase (BARK), the same enzyme that modifies G protein–coupled receptors (see Figure 15-32). β-arrestin then binds to Smo. In turn, β-arrestin recruits the microtubule motor protein Kif7, which binds to the microtubules in the core of the cilium and moves Smo up the ciliary membrane. At the same time, the cytosolic complex containing Gli is disrupted, preventing Gli cleavage into a repressor fragment. Gli subsequently accumulates at the tip of the cilium, a process also requiring the Kif7 motor protein. There it becomes activated by Smo by a mechanism not yet known in detail, and then another motor protein, a dynein, moves the activated Gli, termed Gli*, to the base of the cilium (see Figure 16-34b) As in Drosophila, this active transcription factor then moves into the nucleus, where it activates expressions of multiple target genes.
Inappropriate activation of Hh signaling is the cause of several types of human tumors, including medulloblastomas (cerebellum tumors) and rhabdomyosarcomas (muscle tumors). Primary cilia are essential for this abnormal Hh signaling, and drugs that inhibit the function of primary cilia are being tested on animal models of these cancers. For instance, expression of a mutant activated form of Smoothened in the postnatal mouse brain causes medulloblastomas, but these tumors do not form if, simultaneously, a gene encoding an essential ciliary protein is inactivated.
