Negative Feedback Loops Regulate TGF-β/Smad Signaling
In most signaling pathways, the response to a growth factor or other signaling molecule decreases with time (a phenomenon called desensitization). This response is adaptive because it prevents overreaction and makes fine-tuned control of cellular responses possible. Two cytosolic proteins called SnoN and Ski (Ski stands for “Sloan-Kettering Cancer Institute”) are induced by TGF-β signaling in virtually all body cells and serve to down-modulate the TGF-β/Smad signaling pathway. These proteins were originally identified as cancer-causing oncoproteins because their expression is elevated in many cancers, including melanomas and certain breast cancers, because growth-inhibitory proteins normally induced by TGF-β are not produced. Indeed, when overexpressed in cultured primary fibroblast cells, Ski or SnoN can cause abnormal cell proliferation, and down-regulation of Ski in pancreatic cancers reduces tumor growth.
How SnoN and Ski trigger abnormal cell proliferation was not understood until years later, when they were found to bind to both the co-Smad (Smad4) and phosphorylated R-Smads (Smad3) after TGF-β stimulation. SnoN and Ski do not prevent formation of an R-Smad/co-Smad complex or affect the ability of a Smad complex to bind to DNA regulatory regions. Rather, they block transcription activation by a bound Smad complex, in part by inducing deacetylation of histones in adjacent chromatin segments. This renders the cell resistant to the growth-inhibitory effects of TGF-β (Figure 16-5). The increased levels of these proteins induced by TGF-β are thought to dampen long-term signaling effects due to continued exposure to TGF-β; this is another example of negative feedback, in which a gene induced by TGF signaling, in this case SnoN, inhibits further signaling by TGF-β.
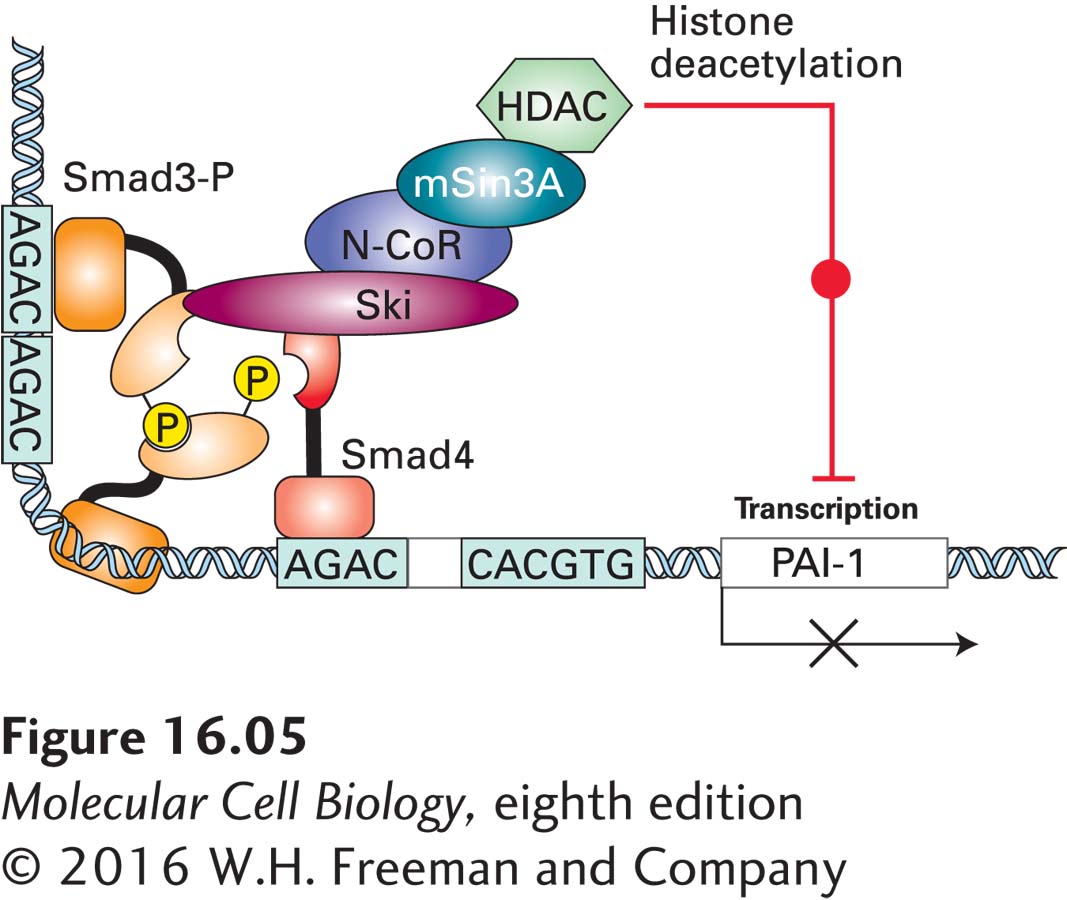
FIGURE 16-5 Model of Ski-mediated down-regulation of Smad transcription-activating function. Ski represses Smad function by binding directly to Smad4. Since the Ski-binding domain on Smad4 significantly overlaps with the Smad4 MH2 domain required for binding the phosphorylated tail of Smad3, binding of Ski disrupts the normal interactions between Smad3 and Smad4 necessary for transcriptional activation. In addition, Ski recruits the protein N-CoR, which binds directly to mSin3A; in turn, mSin3A interacts with histone deacetylase (HDAC), an enzyme that promotes histone deacetylation on nearby promoters, repressing gene expression (see Chapter 9). As a result of both processes, transcription activation induced by TGF-β and mediated by Smad complexes is shut down. The related protein SnoN functions similarly to Ski in repressing TGF-β signaling. See J. Deheuninck and K. Luo, 2009, Cell Res. 19:47.
Among the other proteins induced after TGF-β stimulation are the I-Smads, especially Smad7. Smad7 blocks the ability of activated type I TGF-β receptors (RI) to phosphorylate R-Smad proteins, and Smad7 may also target TGF-β receptors for degradation. In these ways, Smad7, like Ski and SnoN, participates in a negative feedback loop: its induction inhibits intracellular signaling by long-term exposure to the stimulating hormone.
