Microfilaments Function in Endocytosis
As we saw in Chapter 14, endocytosis is the processes that cells use to take up particles, molecules, or fluid from the external medium by enclosing them in plasma membrane and then internalizing them. The uptake of molecules or liquid is called receptor-mediated or fluid-phase endocytosis, and the uptake of large particles is called phagocytosis (“cell eating”). Microfilaments participate in both of these processes.
Fluid-phase endocytosis is a very highly organized process, and recent studies have shown that the power of actin assembly contributes to this mechanism. Endocytosis assembly factors recruit NPFs so that as the endocytic vesicles invaginate and pinch off from the membrane, they are driven into the cytoplasm, powered by a rapid and very short-lived burst (a few seconds in duration) of actin polymerization driven by the Arp2/3 complex (Figure 17-18a). This actin-based movement of endocytic vesicles can be reconstituted in vitro (Figure 17-18b) and is mechanistically very similar to leading-edge formation and Listeria motility.
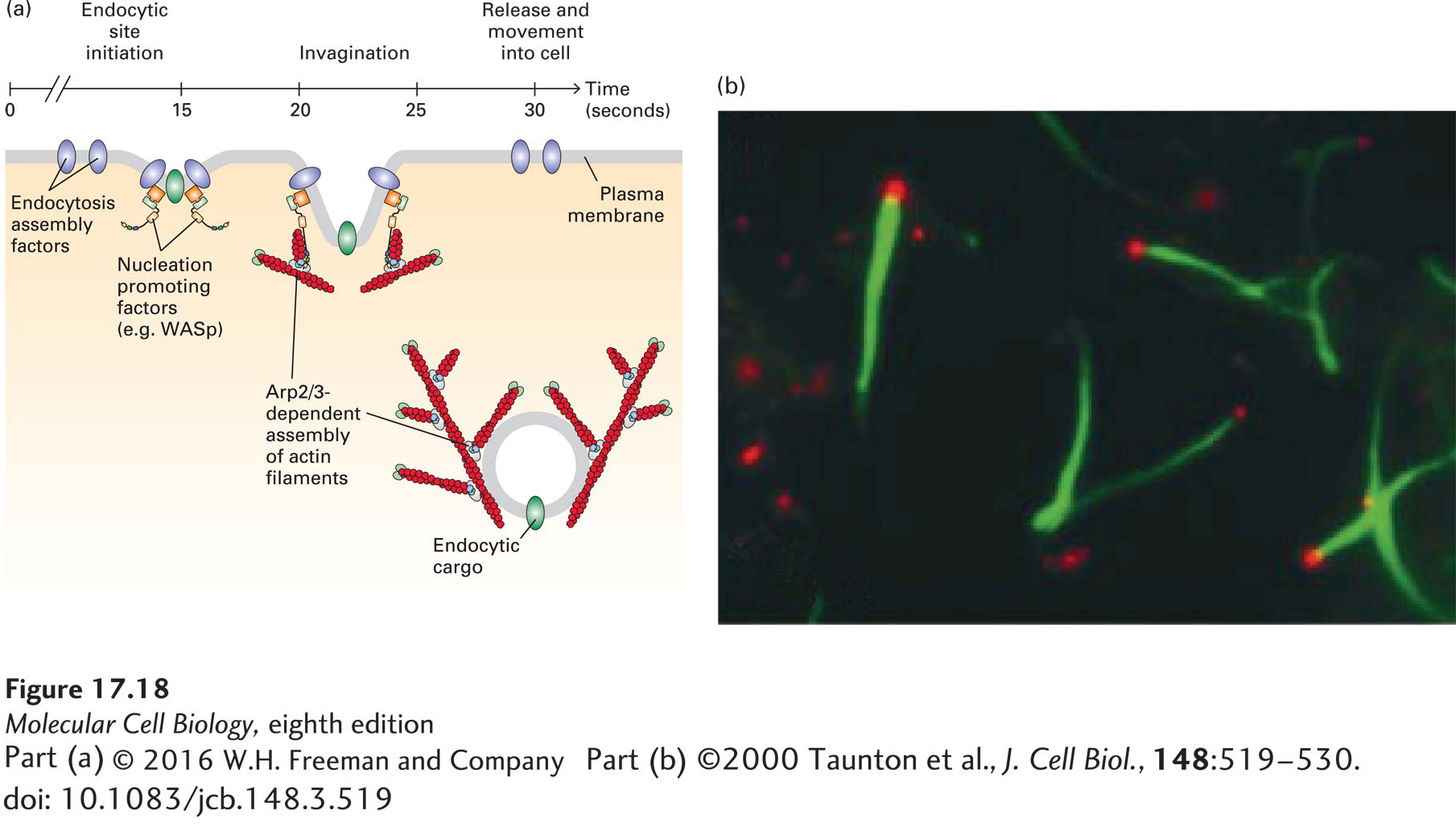
FIGURE 17-18 Arp2/3-dependent actin assembly during endocytosis. (a) Clathrin-mediated endocytosis is a rapid and ordered process. It has been best studied in yeast, in which the temporal order of specific steps has been delineated. In vivo imaging has shown that endocytosis assembly factors recruit NPFs that activate the Arp2/3 complex. A burst of Arp2/3-dependent actin assembly drives internalized endocytic vesicles away from the plasma membrane in a manner similar to the movement of Listeria. (b) Endosome movement can be reconstituted in vitro. Endosomes isolated from cells that had taken up fluorescently labeled transferrin (red) were added to a cell extract containing fluorescently labeled actin (green). The endosomes bind WASp, which then activates the Arp2/3 complex to assemble actin tails that propel them through the cytoplasm.
[Part (b) ©2000 Taunton et al., J. Cell Biol., 148:519–530. doi: 10.1083/jcb.148.3.519.]
Phagocytosis is a vital process in the recognition and removal of pathogens, such as bacteria, by leukocytes (white blood cells). The immune system identifies a bacterium as foreign material and makes antibodies that recognize components on its surface. As we discussed in Chapter 3, each antibody has a region called the Fab domain that binds specifically to its antigen, in this case a component on the bacterial cell surface. As antibodies coat the bacterium through interaction between their Fab domains and the cell-surface antigen, a second antibody domain, known as the Fc domain, is exposed. This process is known as opsonization (Figure 17-19, step 1, see Chapter 23). The leukocytes have a receptor on their cell surface, called the Fc receptor, that recognizes the antibodies on the bacterium; this interaction signals the cells to bind and engulf the pathogen (steps 2 and 3). The signal also tells the cells to assemble microfilaments at the site of interaction with the bacterium, and the assembled microfilaments, together with myosin motor proteins, provide the force necessary to draw the bacterium into the cell, ultimately fully enclosing the pathogen in plasma membrane (step 4). Once internalized, the newly formed phagosome fuses with lysosomes, and the pathogen is killed and degraded by lysosomal enzymes.
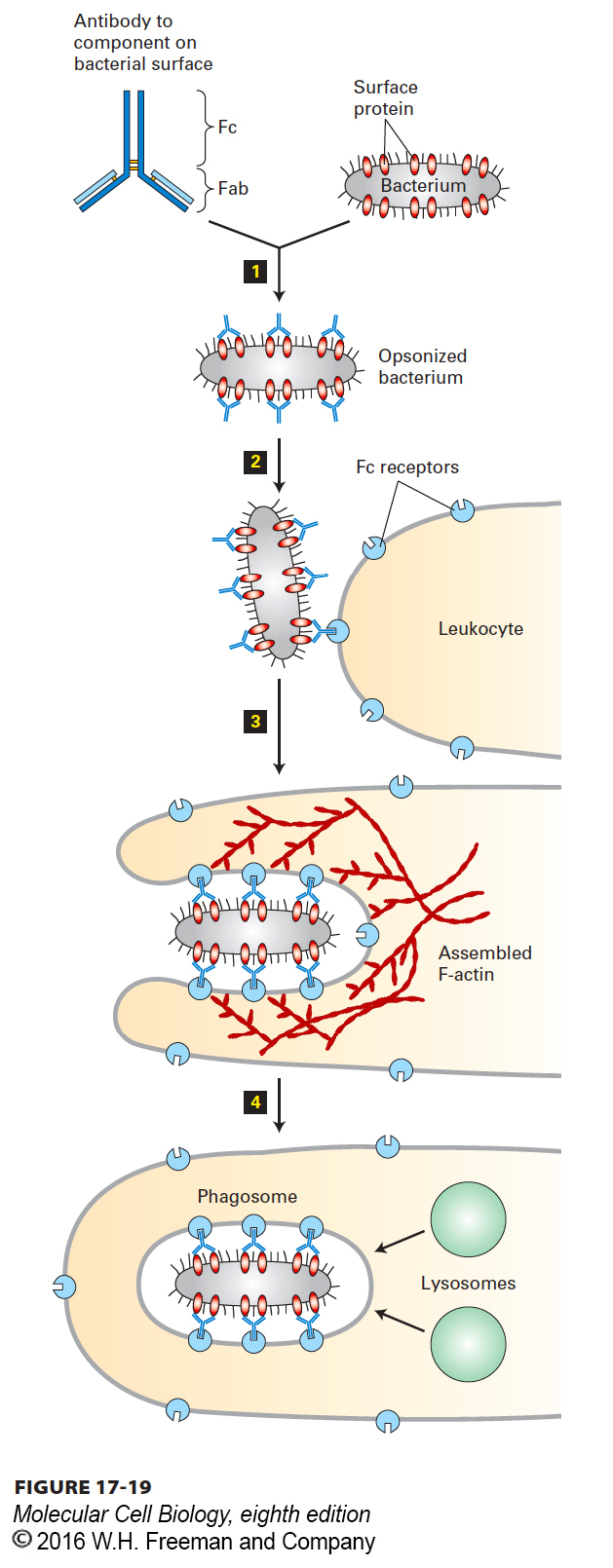
FIGURE 17-19 Phagocytosis and actin dynamics. Actin assembly and contraction drives the phagocytic internalization of particles. Shown here is the phagocytosis and degradation of a bacterium by a leukocyte. An invading bacterium is coated by specific antibodies to a cell-surface protein in a process known as opsonization step 1. The Fc region of the bound antibodies is displayed on the bacterial surface and recognized by a specific receptor, the Fc receptor, on the leukocyte surface step 2. This interaction signals the cell to assemble a contractile actin structure that results in the internalization and engulfment of the bacterium step 3. Once it has been internalized into a phagosome, the bacterium is killed and degraded by enzymes delivered from lysosomes step 4.
Recently, actin assembly has been implicated in other steps of the endocytic pathway as well as in the secretory pathway. For example, an NPF called WASH has been implicated in the Arp2/3-dependent nucleation of actin filament on endosomes, regulating their shape and contributing to transport events. Another NPF, called WHAMM, localizes to the Golgi complex and is believed to direct the Arp2/3-dependent assembly of actin participating in membrane traffic from the endoplasmic reticulum to the Golgi. The emerging view is that actin assembly is not essential for several steps in membrane trafficking, but facilitates transport between different compartments of the secretory and endocytic pathways.

