Cross-Linking Proteins Organize Actin Filaments into Bundles or Networks
When one assembles actin filaments in a test tube, they form a tangled network. In cells, however, actin filaments are found in a variety of distinct structures, such as the highly ordered filament bundles in microvilli or the network characteristic of the leading edge of a motile cell (see Figure 17-4a). These different structures are determined by the filament assembly mechanisms discussed above and by the presence of actin cross-linking proteins. To be able to organize actin filaments, an actin cross-linking protein must have two F-actin–binding sites (Figure 17-20a).
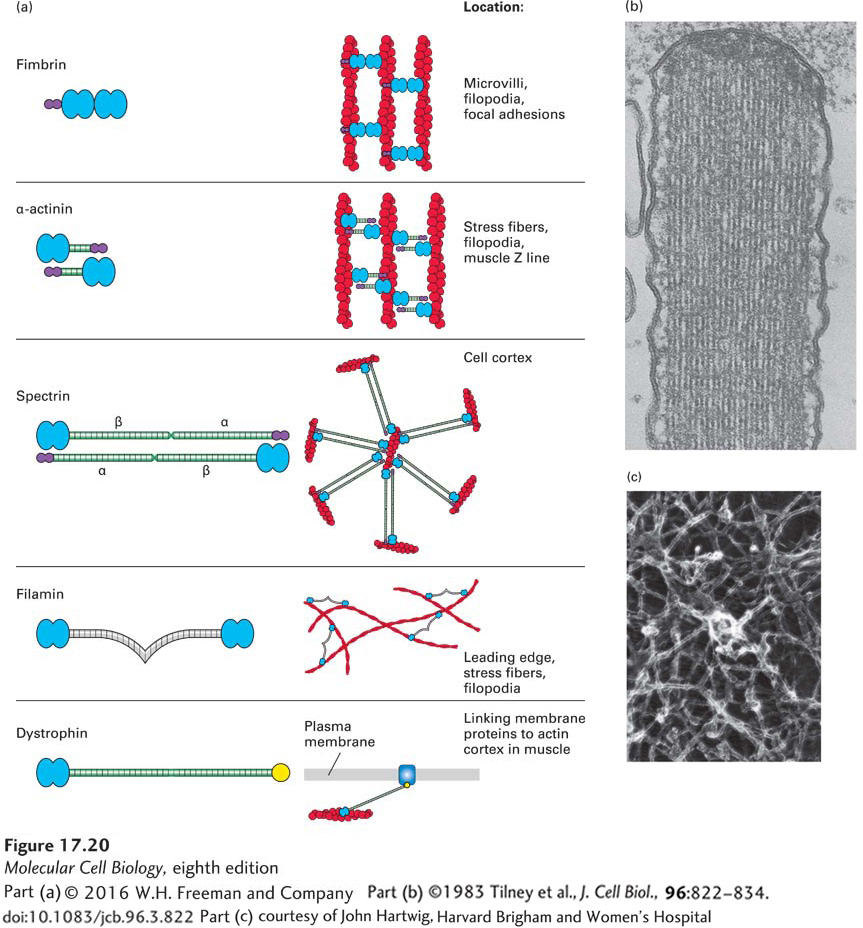
FIGURE 17-20 Actin cross-linking proteins. Actin cross-linking proteins mold F-actin filaments into diverse structures. (a) Examples of four F-actin cross-linking proteins, all of which have two domains (blue) that bind F-actin. Some have a Ca2+-binding site (purple) that inhibits their activity at high levels of free Ca2+. Also shown is dystrophin, which has an actin-binding site on its N-terminal end and a C-terminal domain that binds the membrane protein dystroglycan. (b) Transmission electron micrograph of a thin section of a stereocilium (an unfortunate name, since it is really a giant microvillus) on a sensory hair cell in the inner ear. This structure contains a bundle of actin filaments cross-linked by fimbrin, a small cross-linking protein that allows for close and regular interaction of actin filaments. (c) Long cross-linking proteins such as filamin are flexible and can thus cross-link actin filaments into a loose network.
[Part (b) ©1983 Tilney et al., J. Cell Biol., 96:822–834. doi: 10.1083/jcb.96.3.822; part (c) courtesy of John Hartwig, Harvard Brigham and Women’s Hospital.]
Cross-linking of F-actin can be achieved by having two actin-binding sites within a single polypeptide, as with fimbrin, a protein found in microvilli that builds bundles of filaments all having the same polarity (Figure 17-20b). Other actin cross-linking proteins have a single actin-binding site in a polypeptide chain, and two chains associate to form a dimer that brings together two actin-binding sites. These dimeric cross-linking proteins can assemble to generate a rigid rod connecting the two binding sites, as happens with α-actinin. Like fimbrin, α-actinin bundles parallel actin filaments, but keeps them farther apart than fimbrin. Another protein, called spectrin, is a tetramer with two actin-binding sites; spectrin spans an even greater distance between actin filaments and makes networks under the plasma membrane (shown in Figure 17-21 and discussed in the next section). Other types of cross-linking proteins, such as filamin, have a highly flexible region between the two actin binding sites that functions like a molecular leaf spring, so they can make stabilizing cross-links between filaments in a network (Figure 17-20c) such as that found in the leading edge of a motile cell. The Arp2/3 complex, which we discussed in terms of its ability to nucleate actin filament assembly, is also an important cross-linking protein, attaching the (−) end of one filament to the side of another filament (see Figure 17-15).
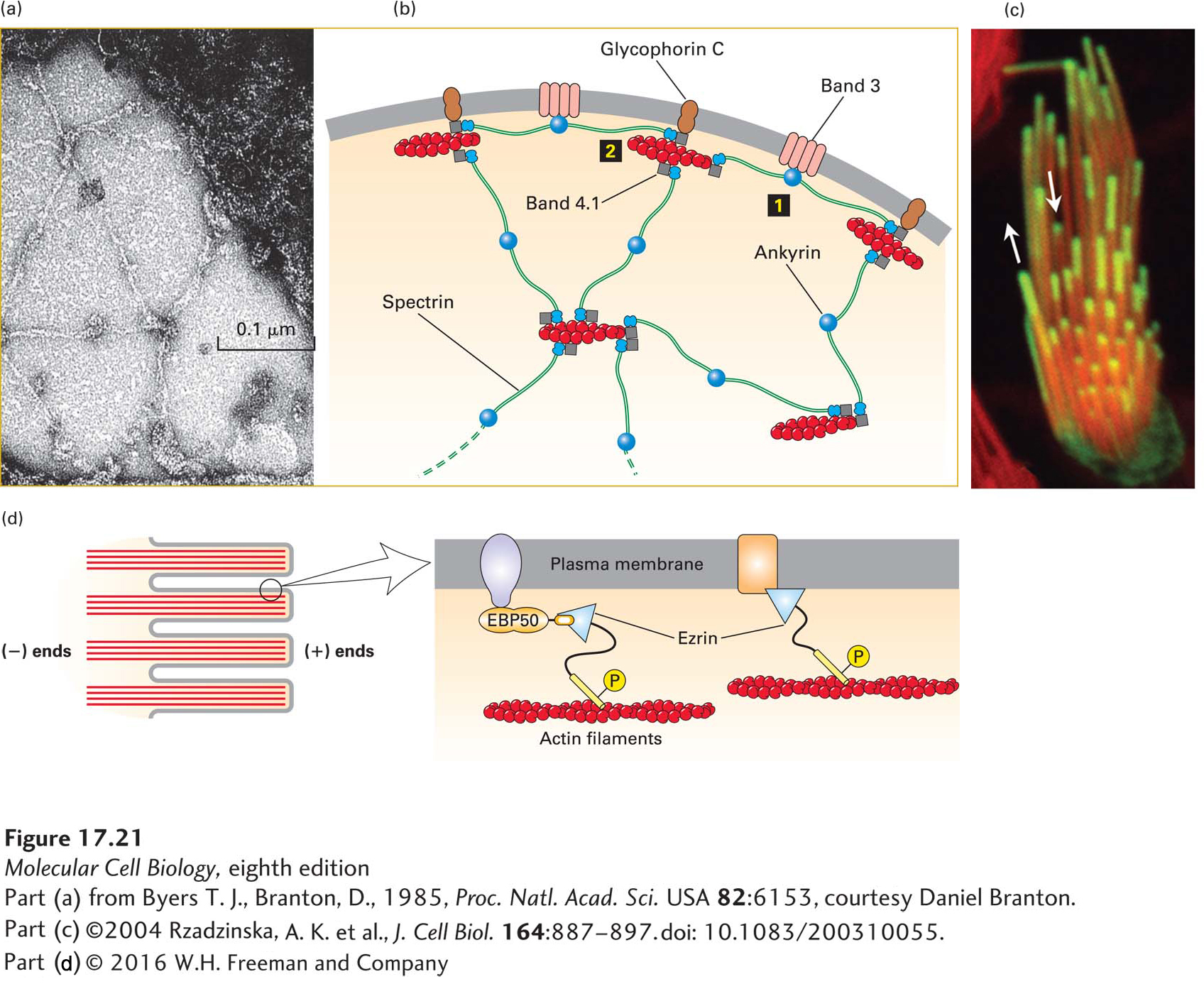
FIGURE 17-21 Lateral attachment of microfilaments to membranes. (a) Electron micrograph of the erythrocyte membrane showing the spoke-and-hub organization of the cortical cytoskeleton supporting the plasma membrane in human erythrocytes. The long spokes are composed mainly of spectrin and can be seen to intersect at the hubs, or membrane-attachment sites. The darker spots along the spokes are ankyrin molecules, which link spectrin to integral membrane proteins. (b) Diagram of the erythrocyte cytoskeleton, showing the two main types of membrane attachments: 1 through ankyrin to band 3 and 2 through band 4.1 to glycophorin C. (c) Actin is incorporated into the tips of stereocilia (giant microvilli). Cells with stereocilia were transfected to express GFP-labeled actin for a short period of time and then counterstained with rhodamine-phalloidin to stain all the F-actin. The experiment shows that new actin is incorporated at the tips of the stereocilia. (d) Ezrin, a member of the ezrin-radixin-moesin (ERM) family, links actin filaments laterally to the plasma membrane in surface structures such as microvilli; attachment can be direct or indirect. Ezrin, activated by phosphorylation (P), links directly to the cytoplasmic region of transmembrane proteins (right) or indirectly through a scaffolding protein such as EBP50 (left). See R. G. Fehon et al., 2010, Nature Rev. Mol. Cell Biol. 11:276, S. E. Lux, 1979, Nature 281:426, and E. J. Luna and A. L. Hitt, 1992, Science 258:955.
[Part (a) from Byers T. J., Branton, D., 1985, Proc. Natl. Acad. Sci. USA 82:6153, courtesy Daniel Branton; part (c) ©2004 Rzadzinska, A. K. et al., J. Cell Biol. 164:887–897. doi: 10.1083/200310055.]

