Myosins Make Up a Large Family of Mechanochemical Motor Proteins
Since all myosins have related S1 domains with considerable similarity in their primary amino acid sequence, it is possible to determine how many myosin genes, and how many different classes of myosins, exist in a sequenced genome. There are about forty myosin genes in the human genome (Figure 17-24), nine in Drosophila, and five in budding yeast. Computer analysis of the sequence relationships between myosin head domains suggests that about 20 distinct classes of myosins have evolved in eukaryotes, with greater sequence similarity within a class than between. As indicated in Figure 17-24, the genetic bases for some conditions have been traced to genes encoding myosins. All myosin head domains convert ATP hydrolysis into mechanical work using the same general mechanism. However, as we will see, subtle variations in this mechanism can have profound effects on the functional properties of different myosin classes. How do these different classes relate with respect to their tail domains? Amazingly, if one takes just the protein sequences of the tail domains of the myosins and uses this information to place them in classes, they fall into the same groupings as the motor domains. This finding implies that head domains with specific properties have coevolved with specific classes of tail domains, which makes a lot of sense, suggesting that each class of myosin has evolved to carry out a specific function.
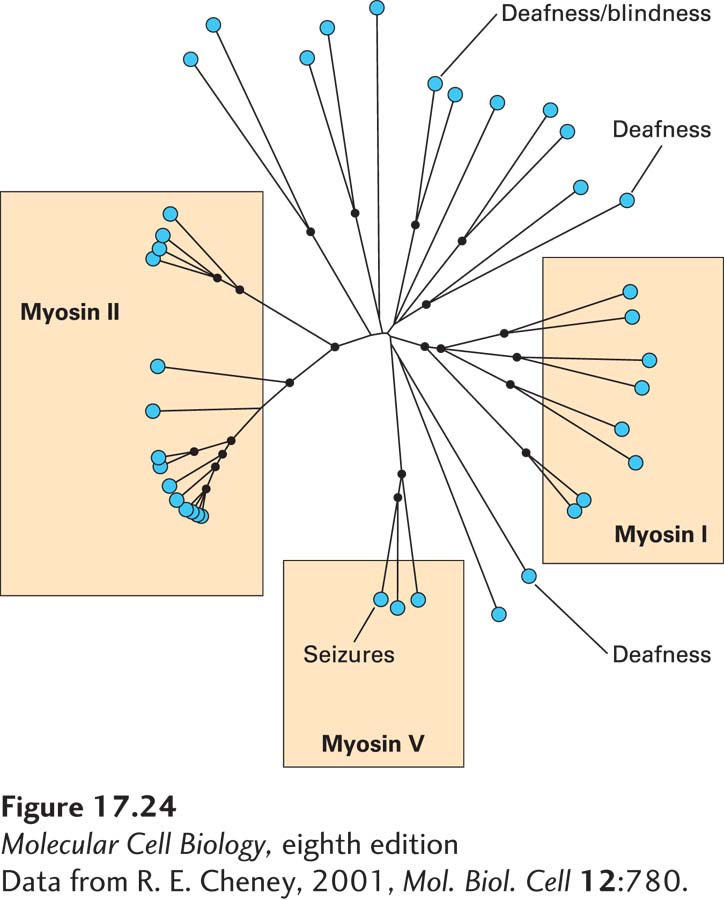
FIGURE 17-24 The myosin superfamily in humans. Results of a computer analysis of the relatedness of the S1 head domains of all of the approximately 40 myosins encoded by the human genome. Each myosin is indicated by a blue dot. The lengths of the black lines indicate phylogenetic distance relationships: myosins connected by short lines are closely related, whereas those separated by longer lines are more distantly related. Among these myosins, three classes—myosins I, II, and V—are widely represented among eukaryotes; others have more specialized functions. Indicated are examples in which loss of a specific myosin causes a disease.
[Data from R. E. Cheney, 2001, Mol. Biol. Cell 12:780.]
Among all these classes of myosins are three especially well-studied ones, which are commonly found in animals and fungi: the so-called myosin I, myosin II, and myosin V families (Figure 17-25). In humans, eight genes encode heavy chains for the myosin I family, fourteen for the myosin II family, and three for the myosin V family (see Figure 17-24).
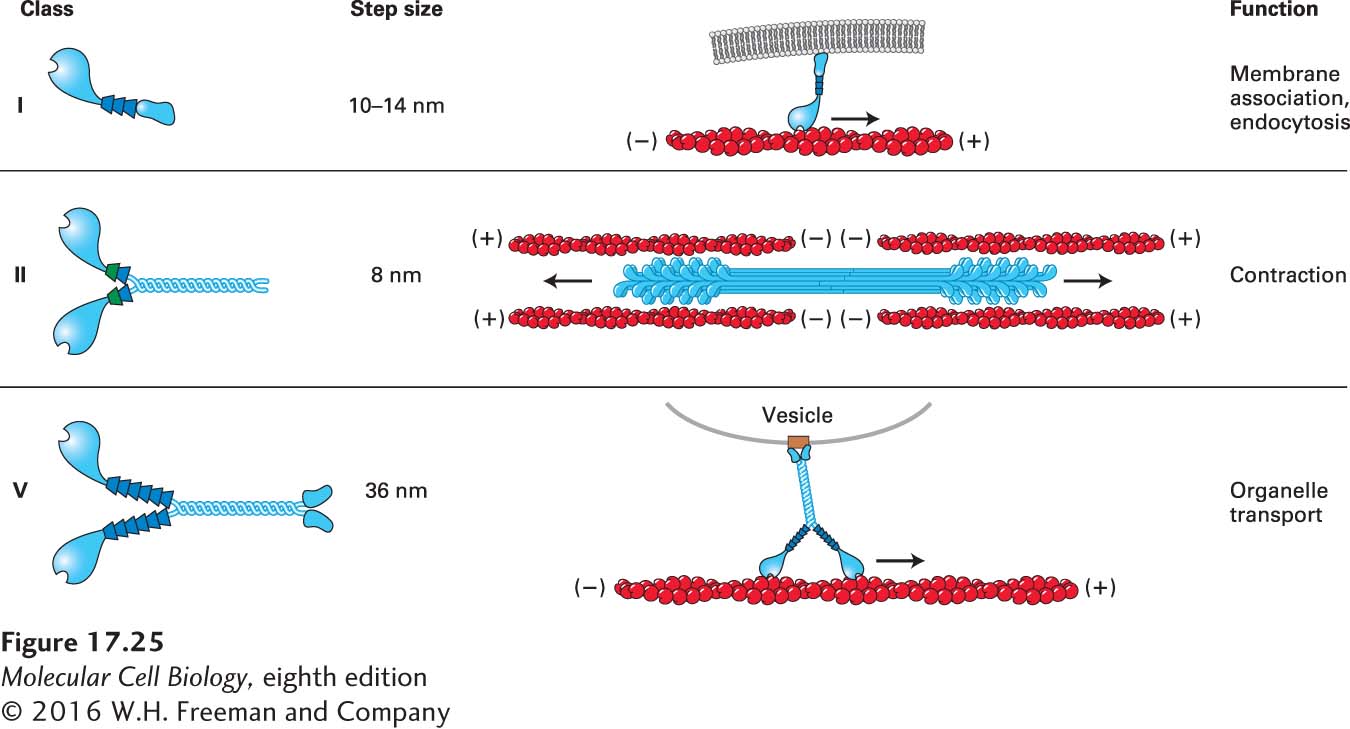
FIGURE 17-25 Three common classes of myosins. Myosin I molecules consist of a head domain and a neck domain; a variable number of light chains is associated with the neck domain. Members of the myosin I class are the only myosins to have a single head domain. Some of these myosins are believed to associate directly with membranes through lipid interactions. Myosin II molecules have two head domains and two light chains per neck and are the only class that can assemble into bipolar filaments. Myosin V molecules have two head domains and six light chains per neck. They bind specific receptors (brown box) on organelles, which they transport. All myosins in these three classes move toward the (+) end of actin filaments.
Myosin II molecules assemble into bipolar filaments with opposite orientations in each half of the filament, so that there is a cluster of head domains at each end of the filament. This organization is important for its involvement in contraction; indeed, this is the only class of myosins involved in contractile functions. The large number of members in this class reflects the need for myosin II filaments with the slightly different contractile properties seen in different muscles (e.g., skeletal, cardiac, and various types of smooth muscle) as well as in nonmuscle cells.
The myosin II class is the only one that assembles into bipolar filaments. All myosin II members have a relatively short neck domain and have two light chains per heavy chain. The myosin I class is quite large, has a variable number of light chains associated with the neck region, and is the only one in which the two heavy chains are not associated through their tail domains and so are single-headed. The large size and diversity of the myosin I class suggests that these myosins perform many functions, most of which remain to be determined, but some members of this family connect actin filaments to membranes, and others are implicated in endocytosis. Since these myosin molecules have only one head each, several of them must work together to generate movement, and at least one must remain attached to an actin filament. Members of the myosin V class have two heavy chains, resulting in a motor with two heads, long neck regions with six light chains each, and tail regions that dimerize and terminate in domains that bind to specific organelles to be transported. As we will see shortly, the length of the neck region affects the rate of myosin movement.
In every case that has been tested so far, myosins move toward the (+) end of an actin filament—with one exception, the myosin VI found in animals. This remarkable myosin has an insert in its head domain to make it work in the opposite direction, and so movement is toward the (−) end of an actin filament. Myosin VI is believed to contribute to endocytosis by moving the endocytic vesicles along actin filaments away from the plasma membrane. Recall that membrane-associated actin filaments have their (+) ends toward the membrane, so a motor directed toward the (−) end would take them away from the membrane toward the center of the cell.

