The structure of the sarcomere is maintained by a number of accessory proteins (Figure 17-32). The actin filaments are stabilized on their (+) ends by CapZ and on their (−) ends by tropomodulin. A giant protein known as nebulin extends along the thin actin filament all the way from the Z disk to tropomodulin, to which it binds. Nebulin consists of repeating domains that bind to the actin in the filament, and it is believed that the number of actin-binding repeats, and therefore the length of nebulin, determines the length of the thin filaments. Another giant protein, called titin (because it is so large), has its head associated with the Z disk and extends to the middle of the thick filament, where another titin molecule extends to the subsequent Z disk. Titin is believed to be an elastic molecule that holds the thick filaments in the middle of the sarcomere and also prevents overstretching to ensure that the thick filaments remain interdigitated between the thin filaments.
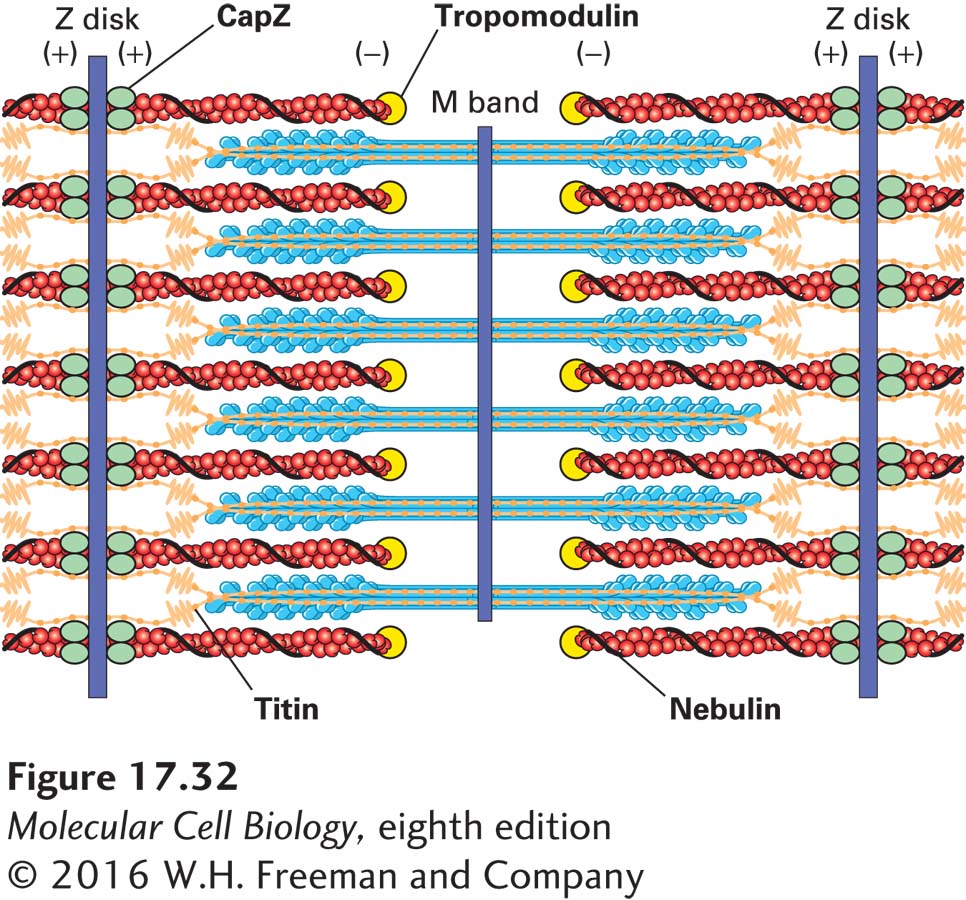
FIGURE 17-32 Accessory proteins found in skeletal muscle. To stabilize the actin filaments, CapZ caps the (+) end of the thin filaments at the Z disk, whereas tropomodulin caps the (−) end. The giant protein titin extends through the thick filaments and attaches to the Z disk. Nebulin binds actin subunits and determines the length of the thin filament.
