Contraction of Skeletal Muscle Is Regulated by Ca2+ and Actin-Binding Proteins
Like many cellular processes, skeletal muscle contraction is initiated by an increase in the cytosolic Ca2+ concentration. As described in Chapter 11, the Ca2+ concentration of the cytosol is normally kept low, below 0.1 µM. In skeletal muscle cells, a low cytosolic Ca2+ level is maintained primarily by a unique Ca2+ ATPase that continually pumps Ca2+ ions from the cytosol containing the myofibrils into the sarcoplasmic reticulum (SR), a specialized endoplasmic reticulum of muscle cells (Figure 17-33). This activity establishes a reservoir of Ca2+ in the SR.
The arrival of a nerve impulse (or action potential; see Chapter 22) at a neuromuscular junction triggers an action potential in the muscle-cell plasma membrane (also known as the sarcolemma). The action potential travels down invaginations of the plasma membrane known as transverse tubules, which penetrate the cell to lie around each myofibril. The arrival of the action potential in the transverse tubules stimulates the opening of voltage-gated Ca2+ channels in the SR membrane, and the ensuing release of Ca2+ from the SR raises the cytosolic Ca2+ concentration in the myofibrils. This elevated Ca2+ concentration induces changes in two accessory proteins, tropomyosin and troponin, which are bound to the actin thin filaments and normally block myosin binding. Changes in the positions of these proteins on the actin thin filaments in turn permit the myosin-actin interactions and hence contraction. This type of regulation is very rapid and is known as thin-filament regulation.
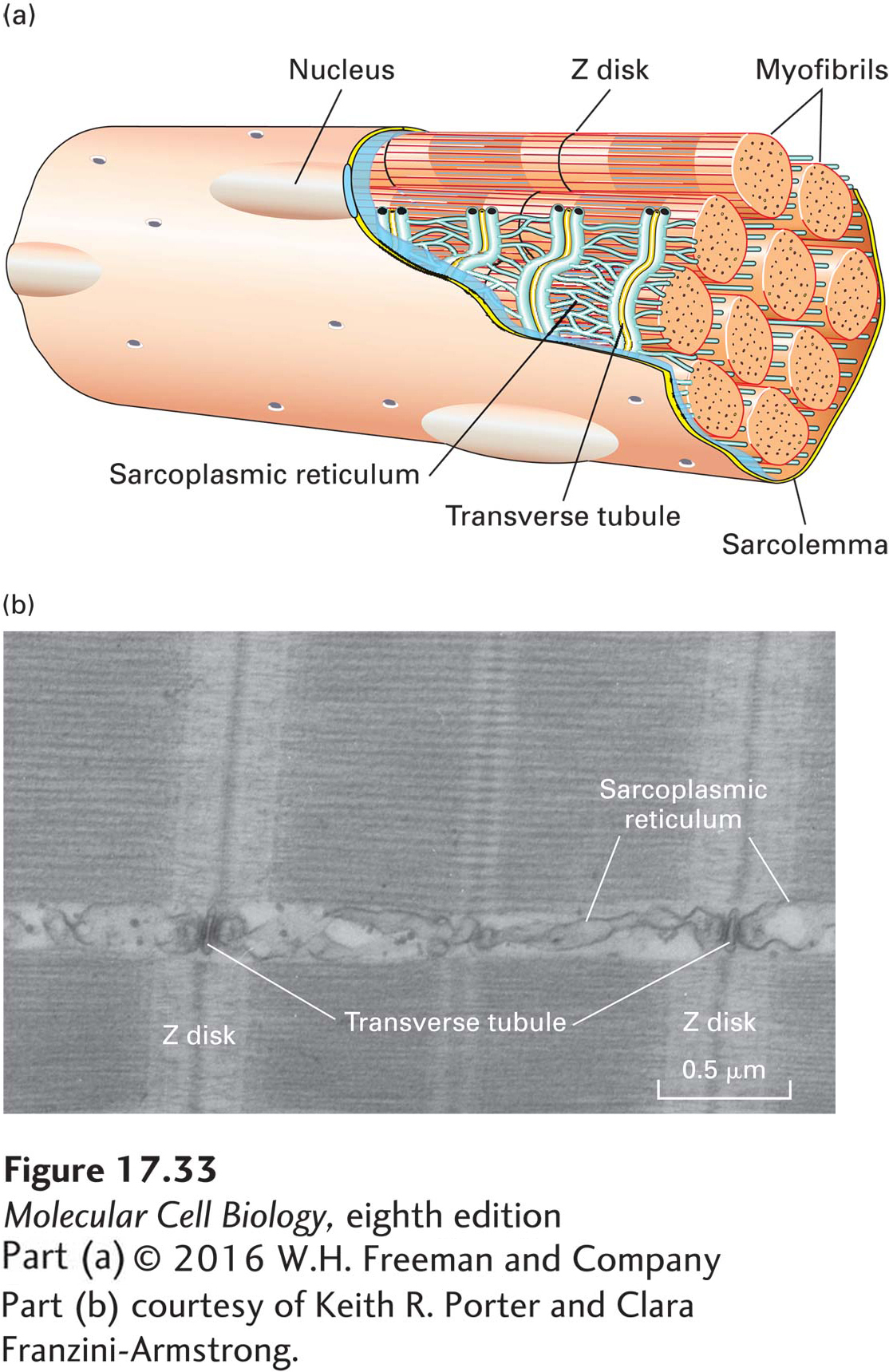
FIGURE 17-33 The sarcoplasmic reticulum regulates the level of free Ca2+ in myofibrils. (a) When a nerve impulse stimulates a muscle cell, the action potential is transmitted down a transverse tubule (yellow), which is continuous with the plasma membrane (sarcolemma), leading to release of Ca2+ from the adjacent sarcoplasmic reticulum (blue) into the myofibrils. (b) Thin-section electron micrograph of skeletal muscle, showing the intimate relationship of the sarcoplasmic reticulum to the muscle fibers.
[Part (b) courtesy of Keith R. Porter and Clara Franzini-Armstrong.]
Tropomyosin (TM) is a ropelike molecule, about 40 nm in length, that binds to seven actin subunits in an actin filament. TM molecules are strung together head to tail, forming a continuous chain along each side of the actin thin filament (Figure 17-34a, b). Associated with each tropomyosin molecule is troponin (TN), a complex of three subunits, TN-T, TN-I, and TN-C. TN-C is the calcium-binding subunit of troponin; it controls the position of TM on the surface of an actin filament through the TN-I and TN-T subunits.
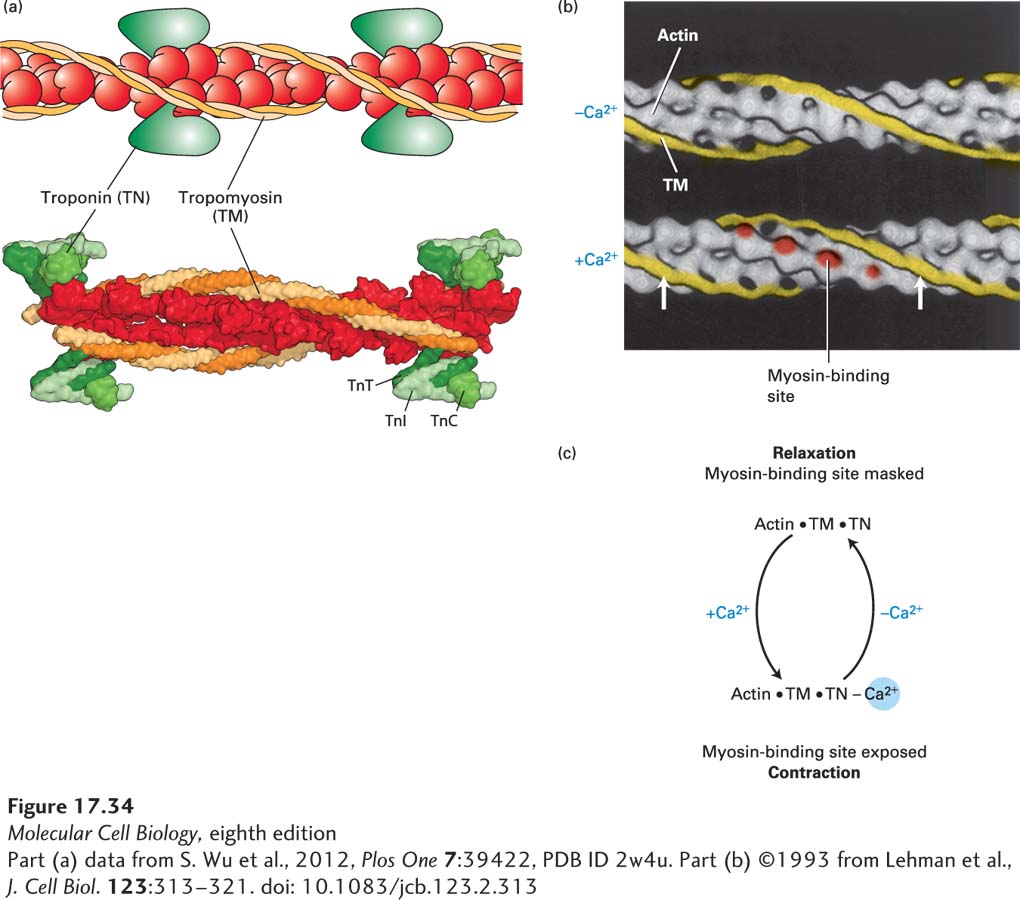
FIGURE 17-34 Ca2+-dependent thin-filament regulation of skeletal muscle contraction. (a) Model and the corresponding structure of the tropomyosin-troponin regulatory complex on a thin filament. Troponin is a protein complex that is bound to the long α-helical tropomyosin molecule. (b) Three-dimensional electron-microscope reconstructions of the tropomyosin helix (yellow) on a muscle thin filament. Tropomyosin in the relaxed state (top) shifts to a new position (arrows) in the state inducing contraction (bottom) when the Ca2+ concentration in the sarcoplasm increases. This movement exposes myosin-binding sites (red) on actin. (Troponin is not shown in this representation, but it remains bound to tropomyosin in both states.) (c) Summary of the regulation of skeletal muscle contraction by Ca2+ binding to troponin.
[Part (a) data from S. Wu et al., 2012, Plos One 7:39422, PDB ID 2w4u. Part (b) ©1993 from Lehman et al., J. Cell Biol. 123:313–321. doi: 10.1083/jcb.123.2.313]
Under the control of Ca2+ and TN, TM can occupy either of two positions on a thin filament. In the absence of Ca2+, TM blocks myosin’s interaction with F-actin, and the muscle is relaxed. Binding of Ca2+ ions to TN-C triggers movement of TM to a new position on the filament, thereby exposing the myosin-binding sites on actin (see Figure 17-34b). Thus, at Ca2+ concentrations greater than 1 µM, the inhibition exerted by the TM-TN complex is relieved, and contraction occurs. The Ca2+-dependent cycling between relaxation and contraction states in skeletal muscle is summarized in Figure 17-34c.
Heart muscle, like skeletal muscle, is subject to thin-filament regulation, using cardiac-specific tropomyosin and troponin. During a heart attack (myocardial infarction), heart cells are deprived of sufficient oxygen (cardiac ischemia) and may subsequently die. The plasma membrane of the dead cells ruptures and releases cellular components into the bloodstream. Blood tests that specifically measure the level of cardiac-specific troponins are used by physicians to determine the severity of a heart attack.

