Myosin-Dependent Mechanisms Regulate Contraction in Smooth Muscle and Nonmuscle Cells
Smooth muscle is a specialized tissue composed of contractile cells that is found in many internal organs. For example, smooth muscle surrounds blood vessels to regulate blood pressure, surrounds the intestine to move food through the gut, and restricts airway passages in the lung. Smooth muscle cells contain large, loosely aligned contractile bundles that resemble those in epithelial cells. The contractile apparatus of smooth muscle and its regulation constitute a valuable model, as its contractile activity is regulated in a manner similar to that in nonmuscle cells. As we have just seen, skeletal muscle contraction is regulated by the switching of the tropomyosin-troponin complex bound to the actin thin filament between the contraction-inducing state in the presence of Ca2+ and the relaxed state in its absence. In contrast, smooth muscle contraction is regulated by the cycling of myosin II between on and off states. Myosin II cycling, and thus contraction of smooth muscle and nonmuscle cells, is regulated in response to many extracellular signaling molecules.
Contraction of vertebrate smooth muscle is regulated primarily by a pathway in which the myosin regulatory light chain (LC) associated with the myosin II neck domain (see Figure 17-22b) undergoes phosphorylation and dephosphorylation. When the regulatory LC is not phosphorylated, the smooth muscle myosin II adopts a folded conformation, and its ATPase cycle is inactive. When the regulatory LC is phosphorylated by the enzyme myosin light-chain kinase (MLC kinase), whose activity is regulated by the level of cytosolic free Ca2+, the myosin II unfolds, assembles into active bipolar filaments, and becomes active to induce contraction (Figure 17-36). The Ca2+-dependent regulation of MLC kinase activity is mediated through the Ca2+-binding protein calmodulin (see Figure 3-33). Calcium first binds to calmodulin, which induces a conformational change in the protein, and the Ca2+/calmodulin complex then binds to MLC kinase and activates it. When the Ca2+ returns to its resting level, MLC kinase becomes inactive, and myosin light-chain phosphatase removes the phosphates to allow the system to return to its relaxed state. This mode of regulation relies on the diffusion of Ca2+ over greater distances than in sarcomeres and on the action of protein kinases, so contraction is much slower in smooth muscle than in skeletal muscle. Because this regulation involves myosin, it is known as thick-filament regulation.
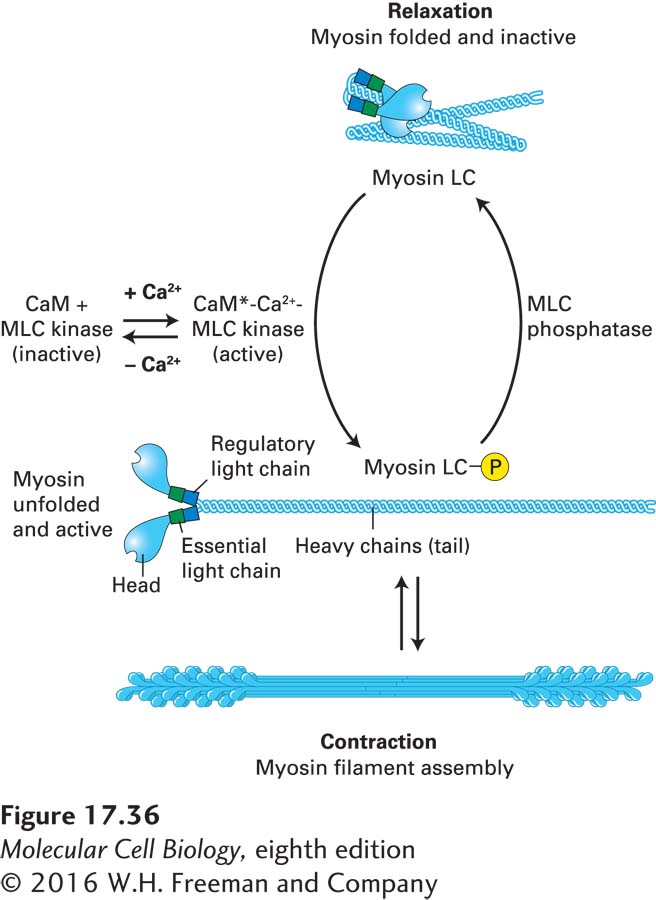
FIGURE 17-36 Myosin light-chain phosphorylation regulates smooth muscle contraction. In vertebrate smooth muscle, phosphorylation of the myosin regulatory light chain (LC) activates contraction. At Ca2+ concentrations of less than 10−6 M, the regulatory light chain is not phosphorylated, and the myosin adopts a folded conformation. When the Ca2+ level rises, Ca2+ binds calmodulin (CaM), which undergoes a conformational change (CaM*). The CaM*-Ca2+ complex binds and activates myosin light-chain kinase (MLC kinase), which then phosphorylates the myosin LC. This phosphorylation event unfolds the myosin II, which is now active and can assemble into bipolar filaments to participate in contraction. When the Ca2+ levels drop, the myosin LC is dephosphorylated by myosin light-chain (MLC) phosphatase, which is not dependent on Ca2+ for activity, causing muscle relaxation.
Unlike skeletal muscle, which is stimulated to contract solely by nerve impulses, smooth muscle cells and nonmuscle cells are regulated by many types of external signals. For example, norepinephrine, angiotensin, endothelin, histamine, and other signaling molecules can modulate or induce the contraction of smooth muscle or elicit changes in the shape and adhesion of nonmuscle cells by triggering various signal transduction pathways. Some of these pathways lead to an increase in the cytosolic Ca2+ concentration; as previously described, this increase can stimulate myosin activity by activating MLC kinase (see Figure 17-36). As we will see below, other pathways activate Rho kinase, which is also able to activate myosin activity by phosphorylating the regulatory light chain, although in a Ca2+-independent manner.
