Classic Experiment 17-2: Sensing Chemotactic Gradients
Sensing Chemotactic Gradients
To investigate how Dictyostelium amoebae sense a chemotactic gradient, investigators have studied the cell-surface receptors for extracellular cAMP and downstream signaling pathways in the expectation that these components must somehow sense the concentration gradient. Before we discuss the details, let’s consider how such a system might work. If a cell can sense a 2 percent difference in concentration across its length, it is unlikely that simply activating actin assembly 2 percent more at the front than at the back of the cell could lead to directed movement. Rather, there must be some mechanism that amplifies this small external signal difference into a large internal biochemical difference. One way to do this would be for the cell to subtract the average signal from the front and back and respond only to a difference in signal. It is believed that this is how the system works. To try to understand this mechanism, investigators have looked at the concentrations of active components of the signaling pathway to see where the amplification occurs.
Micrographs of cAMP receptors tagged with green fluorescent protein (GFP) showed that the receptors are distributed uniformly on the surface of an amoeba cell (Figure 1a); therefore, an internal gradient must be established by another component of the signaling pathway. Because cAMP receptors signal through trimeric G proteins (see Chapter 16), a subunit of the trimeric G protein and other downstream signaling proteins were tagged with GFP to look at their distributions. Fluorescence micrographs showed that the concentration of trimeric G proteins is also rather uniform. Downstream of the trimeric G proteins is PI-3 kinase, an enzyme that phosphorylates membrane-bound inositol phospholipids (phosphoinositides) such as phosphatidylinositol 4,5-biphosphate [PI(4,5)P2], creating the signaling lipid phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] (see Figure 16-25). Remarkably, the enzyme PI-3 kinase is highly enriched at the front of a migrating cell, as are its products. PTEN, the phosphatase that dephosphorylates the signaling lipid PI(3,4,5)P3 back to PI(4,5)P2, is enriched in the tail of the migrating cell (Figure 1b).
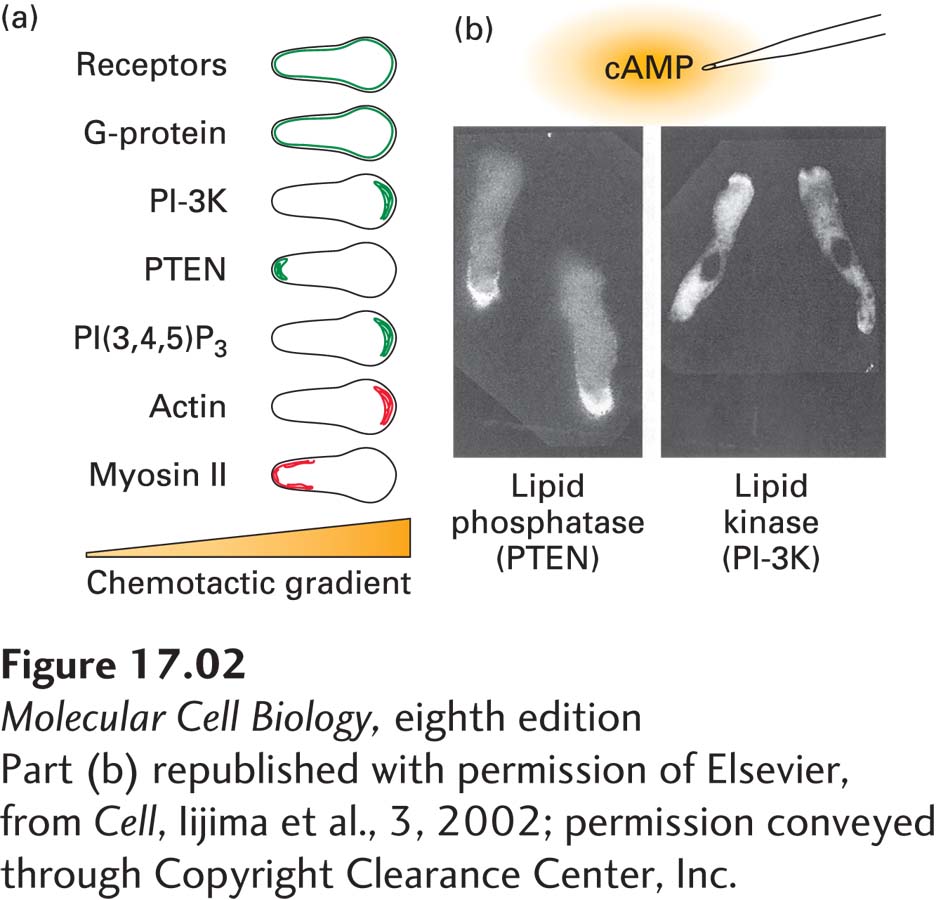
FIGURE 1 Chemotaxis involves elevated levels of the signaling phospholipid PI(3,4,5)P3, which signals to the actin cytoskeleton. (a) Summary of results of studies exploring the localization of components of signaling pathways (green) in Dictyostelium cells undergoing chemotaxis toward cAMP. Also shown are the localization of actin and myosin (red). (b) The enzyme PI-3 kinase, which generates PI(3,4,5)P3, is enriched at the front of chemotaxing cells, whereas PTEN, the phosphatase that hydrolyzes PI(3,4,5)P3, is enriched at the back. These distributions result in elevated PI(3,4,5)P3 at the front of the cells, which signals the polarity for movement.
[Part (b) republished with permission of Elsevier, from Cell, Iijima et al., 3, 2002; permission conveyed through Copyright Clearance Center, Inc.]
This asymmetry is believed to be established in the following way. Prior to the cell’s exposure to a cAMP gradient, the phosphatase PTEN is associated uniformly with the plasma membrane. When a cell “sees” a gradient, PI-3 kinase is activated a bit more at the front than at the back. This results in slightly higher levels of the signaling phospholipid at the front and, through this and other pathways, a reduction of PI(4,5)P2. The association of the phosphatase PTEN with the membrane is very sensitive to the level of PI(4,5)P2, so it is preferentially depleted from the front. Since it is less effective at dephosphorylating the PI(3,4,5)P3 at the front and more effective at dephosphorylating PI(3,4,5)P3 at the rear, a strong asymmetry of PI(3,4,5)P3 results. Thus the phosphatase PTEN contributes to the background subtraction necessary for a cell to sense a shallow gradient of chemoattractant.
The difference in local PI(3,4,5)P3 concentration now signals to the actin cytoskeleton to assemble a leading edge at the front and contraction machinery at the rear (see Figure 1a), and the cell is on its way to the source of chemoattractant. A very similar mechanism has been implicated in the chemotaxis of leukocytes. This cell polarization is not stable in the absence of the chemotactic gradient, so if the gradient changes, as might happen with a leukocyte chasing a moving bacterium, the cell also changes its direction and follows the gradient to its source.
While PI(3,4,5)P3 is clearly important in chemotaxis, Dictyostelium cells have been generated in which the genes encoding each of the five PI-3 kinases normally present in these cells have been disrupted. These cells can still follow a concentration gradient, but not as well as wild-type cells. Thus, as is often the case for fundamental processes, redundant signaling pathways are involved in chemotaxis, and researchers are now busily investigating their molecular basis.
