Actin Filaments Grow Faster at (+) Ends Than at (−) Ends
We saw earlier that myosin S1 decoration experiments revealed an inherent structural polarity of F-actin that is due to the uniform subunit orientation in the filament (see Figure 17-6). If free ATP–G-actin is added to a preexisting myosin-decorated filament, the two ends grow at very different rates (Figure 17-9). In fact, the rate of addition of ATP–G-actin is nearly 10 times faster at the (+) end than at the (−) end. The rate of addition is, of course, determined by the concentration of free ATP–G-actin. Kinetic experiments have shown that the rate of addition is about 12 µM−1 s−1 at the (+) end and about 1.3 µM−1 s−1 at the (−) end (Figure 17-10a). This means that if 1 µM of free ATP–G-actin is added to preformed filaments, 12 subunits, on average, will be added to the (+) end every second, whereas only 1.3 will be added to the (−) end every second. What about the rate of subunit loss from each end? By contrast, the rates of dissociation of ATP–G-actin subunits from the two ends are quite similar, about 1.4 s−1 from the (+) end and 0.8 s−1 from the (−) end. Since this dissociation is simply the rate at which subunits leave ends, it does not depend on the concentration of free ATP–G-actin.
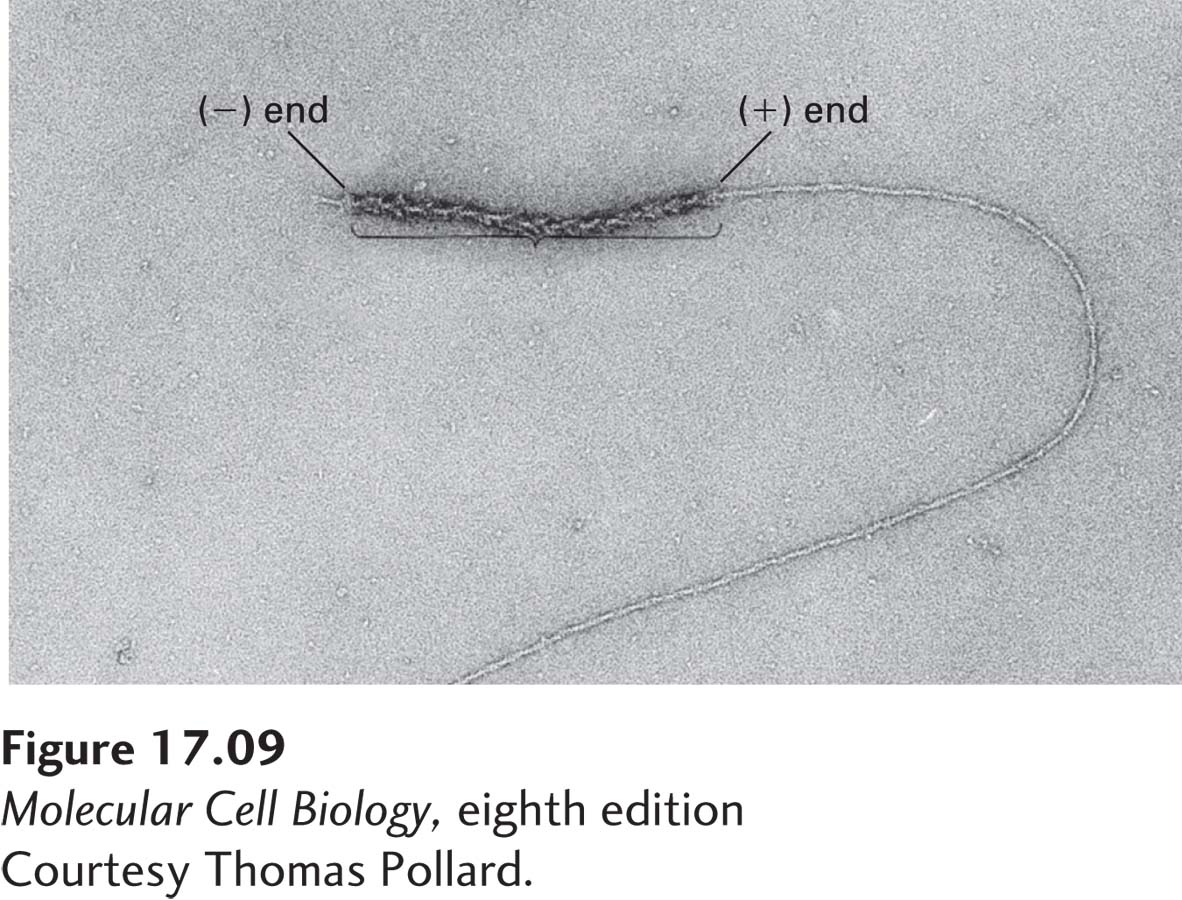
EXPERIMENTAL FIGURE 17-9 The two ends of a myosin-decorated actin filament grow at different rates. When short actin filaments are decorated with myosin S1 heads to reveal the orientation of the filaments and then used to nucleate actin polymerization, G-actin monomers are added much more efficiently to the (+) ends than to the (−) ends of the nucleating filaments.
[Courtesy Thomas Pollard.]
What implications do these association and dissociation rates have for actin dynamics? First, let’s consider just one end, the (+) end. As we noted above, the rate of addition of subunits depends on the free ATP–G-actin concentration, whereas the rate of loss of subunits does not. Thus subunits will be added at high free ATP–G-actin concentrations, but as the concentration is lowered, a point will be reached at which the rate of addition is balanced by the rate of loss, and no net growth occurs at that end. This point is called the critical concentration C+c for the (+) end, and we can calculate it by setting the rate of assembly equal to the rate of disassembly. Thus, at the critical concentration, the rate of assembly is C+c times the measured rate of addition of 12 µM−1 s−1 (C+c 12 s−1), whereas the rate of disassembly is independent of the free actin concentration, namely, 1.4 s−1. Setting these two rates equal to each other yields C+c = 1.4 s−1/12 µM−1 s−1, or 0.12 µM, for the (+) end. Above this free ATP–G-actin concentration, subunits are added to the (+) end and net growth occurs, whereas below it, there is a net loss of subunits, and shrinkage occurs.
Now let’s consider just the (−) end. Because the rate of addition is much lower, 1.3 µM−1 s−1, yet the rate of dissociation is about the same, 0.8 s−1, we expect the critical concentration C–c at the (−) end to be higher than C+c. Indeed, as we just did for the (+) end, we can calculate C–c to be about 0.8 s−1/1.3 µM−1 s−1, or 0.6 µM. Thus, at less than 0.6 µM free ATP–G-actin—say, 0.3 µM—the (−) end will lose subunits. But notice that at this concentration the (+) end will grow, because 0.3 µM is above C+c. Because the critical concentrations for the two ends are different, at steady state the free ATP–G-actin will be intermediate between C+c and C–c, so the (+) end will grow and the (−) end will lose subunits. This phenomenon is known as treadmilling because particular subunits, such those shown in blue in Figure 17-10b, appear to move through the filament.
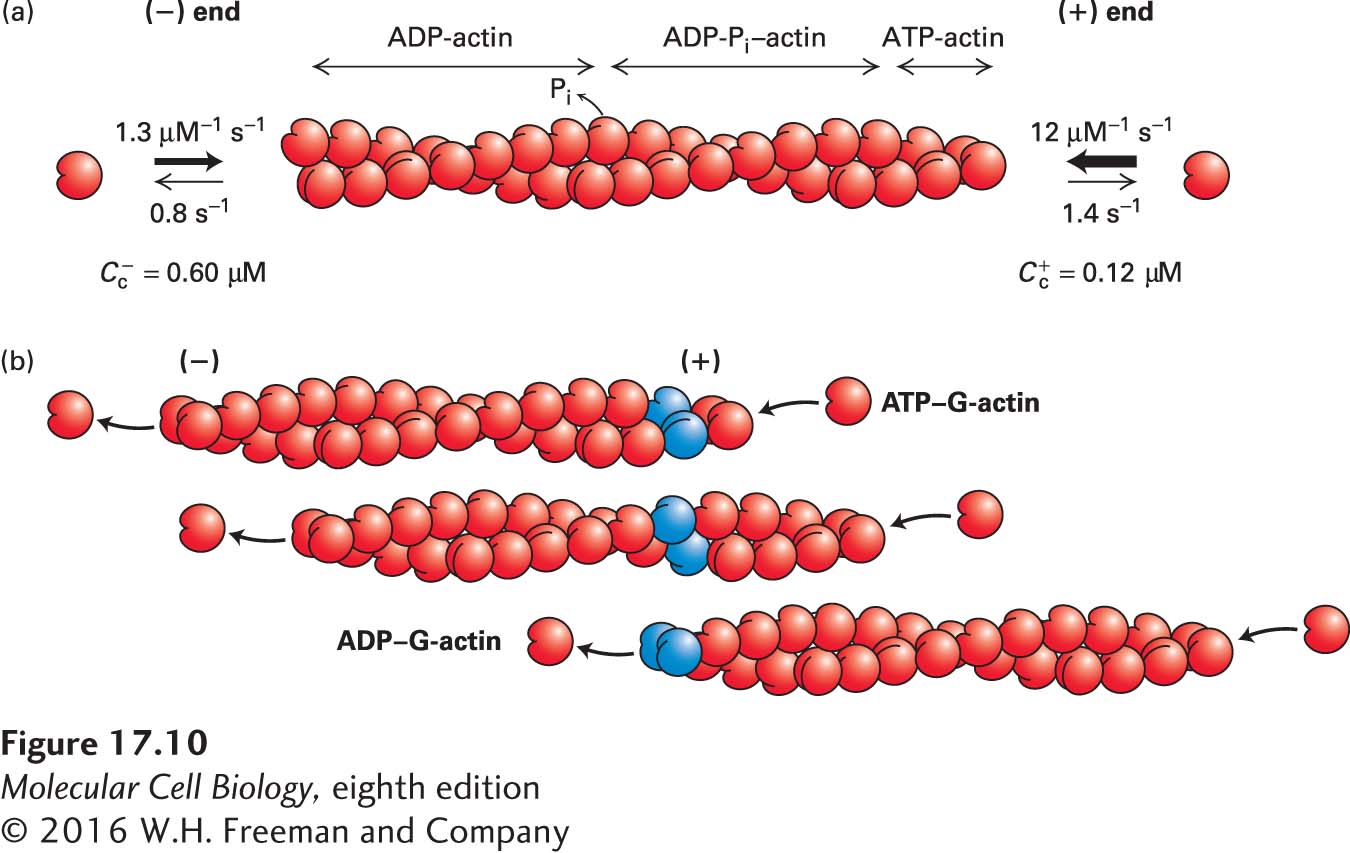
FIGURE 17-10 Actin treadmilling. ATP-actin subunits are added faster at the (+) end than at the (−) end of an actin filament, resulting in a lower critical concentration at the (+) end and treadmilling at steady state. (a) The rate of addition of ATP–G-actin is much faster at the (+) end than at the (−) end, whereas the rate of dissociation of ADP–G-actin is similar at the two ends. This difference results in a lower critical concentration at the (+) end. At steady state, ATP-actin is added preferentially at the (+) end, giving rise to a short region of the filament containing ATP-actin and regions containing ADP-Pi–actin and ADP-actin toward the (−) end. (b) At steady state, ATP–G-actin subunits add preferentially to the (+) end, while ADP–G-actin subunits disassemble from the (−) end, giving rise to treadmilling of subunits. Two actin subunits are colored blue as a reference point within the filament.
The treadmilling of actin filaments is powered by hydrolysis of ATP. When ATP–G-actin binds to a (+) end, ATP is hydrolyzed to ADP and Pi. The Pi is slowly released from the subunits in the filament, so that the filament becomes asymmetric, with ATP-actin subunits at the (+) end followed by a region with ADP-Pi–actin and then, after Pi release, ADP-actin subunits toward the (−) end (see Figure 17-10a). During hydrolysis of ATP and the subsequent release of Pi from subunits in a filament, actin undergoes a conformational change that is responsible for the different association and dissociation rates at the two ends. Here we have considered only the kinetics of ATP–G-actin, but in reality it is ADP–G-actin that dissociates from the (−) end. Our analysis also relies on a plentiful supply of ATP–G-actin, which, as we will see, turns out to be the case in vivo. Thus actin can use the power generated by hydrolysis of ATP to treadmill, and treadmilling filaments can do work in vivo, as we will see later.
How are the amounts of ATP-actin, ADP-Pi-actin, and ADP-actin in a filament determined? The actin ATPase activity and Pi release are slow, so when the rate of actin assembly is enhanced, the amount of ATP-actin at the (+) end is also enhanced. As we will see in the following section, cells have mechanisms to regulate the assembly, as well as the disassembly, of filaments, thereby regulating the nucleotide distribution along a filament.

