The Kinesins Form a Large Protein Superfamily with Diverse Functions
Following the discovery of kinesin-1, a number of proteins with similar motor domains were identified by both genetic screens and molecular biology approaches. There are now 14 known classes of kinesins in animals, defined as sharing amino acid sequence homology with the motor domain of kinesin-1. Proteins of the kinesin superfamily are encoded by about 45 genes in the human genome. Although the functions of all these proteins have not yet been elucidated, some of the best-studied kinesins are involved in processes such as organelle, mRNA, and chromosome transport, microtubule sliding, and microtubule depolymerization.
As with the different classes of myosin motors, in the various kinesin families the conserved motor domain is fused to a variety of class-specific nonmotor domains (Figure 18-20). Whereas kinesin-1 has two identical heavy chains and two identical light chains, members of the kinesin-2 family (also involved in organelle transport) have two different related heavy-chain motor domains and a third polypeptide that associates with the tail and binds cargo. Members of the bipolar kinesin-5 family have four heavy chains, forming bipolar motors that can cross-link antiparallel microtubules and, by walking toward the (+) end of each microtubule, slide them past each other. The kinesin-14 motor proteins are the only known class to move toward the (−) end of a microtubule; this class functions in mitosis. Members of the kinesin-13 family have two subunits, but with the conserved kinesin domain in the middle of the polypeptide. Kinesin-13 proteins do not have motor activity; instead, they are special ATP-hydrolyzing proteins that can enhance the depolymerization of microtubule ends (see Figure 18-15).
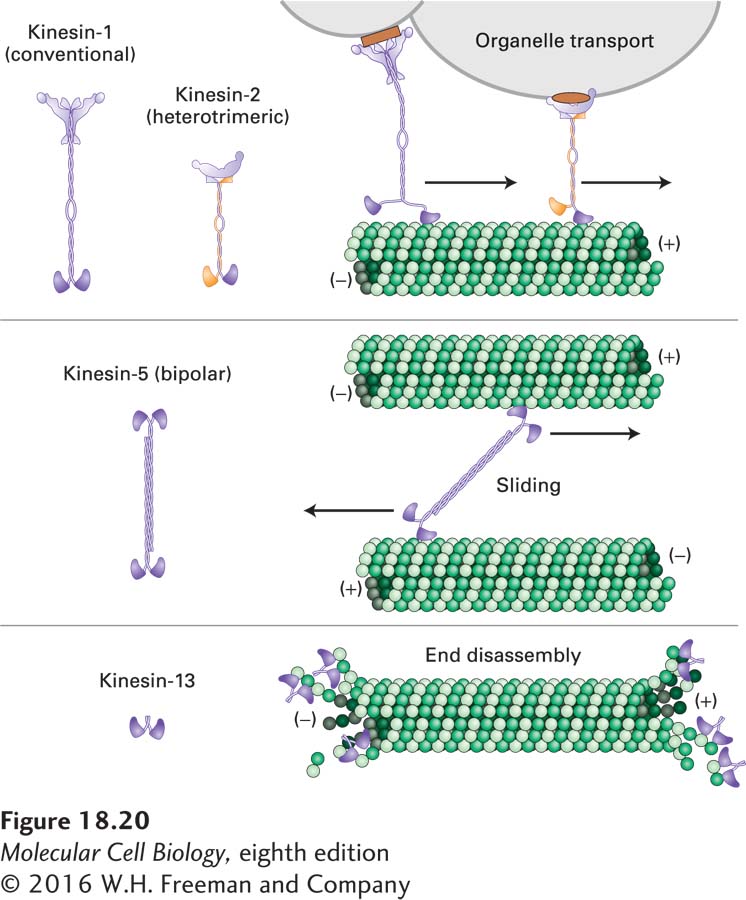
FIGURE 18-20 Structure and function of selected members of the kinesin superfamily. Kinesin-1, which includes the original kinesin isolated from squid axons, is a (+) end–directed microtubule motor involved in organelle transport. The kinesin-2 family has two different, but closely related, heavy chains and a third cargo-binding subunit; this class also transports organelles in a (+) end–directed manner. Members of the kinesin-5 family have four heavy chains assembled in a bipolar configuration to interact with two antiparallel microtubules and also move toward the (+) ends. Kinesin-13 family members have the motor domain in the middle of their heavy chains and do not have motor activity, but they do destabilize microtubule ends (see also Figure 18-15a). Additional kinesin family members are mentioned in the text. Different kinesins have been given many different names; we use the unified nomenclature described in C. J. Lawrence et al., 2004, J. Cell Biol. 167:19–22. See R. D. Vale, 2003, Cell 112:467.

