Dynein Motors Transport Organelles Toward the (−) Ends of Microtubules
In addition to kinesin motors, which primarily mediate anterograde (+) end–directed transport of organelles, cells use another motor protein, cytoplasmic dynein, to transport organelles in a retrograde direction toward the (−) ends of microtubules. This motor protein is very large, consisting of two large (>500 kDa), two intermediate, and two small subunits. It is responsible not only for the ATP-dependent retrograde transport of organelles toward the (−) ends of microtubules in axons, but for many other functions we will consider in the following sections. Compared with the myosins and the kinesins, the family of dynein-related proteins is not very diverse.
Like kinesin-1, cytoplasmic dynein is a two-headed molecule, built around two identical or nearly identical heavy chains. However, because of the enormous size of the motor domain, dynein has been less well characterized in terms of its mechanochemical activity. A single dynein heavy chain consists of a number of distinct domains (Figure 18-24a). The first is the stem, to which the other dynein subunits bind, and which associates with cargo through another protein complex, dynactin. The next part of the heavy chain is a linker that plays a critical role during ATP-dependent motor activity. A large part of the heavy chain makes up the head containing the AAA ATPase domain, consisting of six repeats that assemble into a flowerlike structure, within which lies the ATPase activity. Embedded between the fourth and fifth AAA repeats is the stalk, which protrudes from the structure and contains the microtubule-binding region.
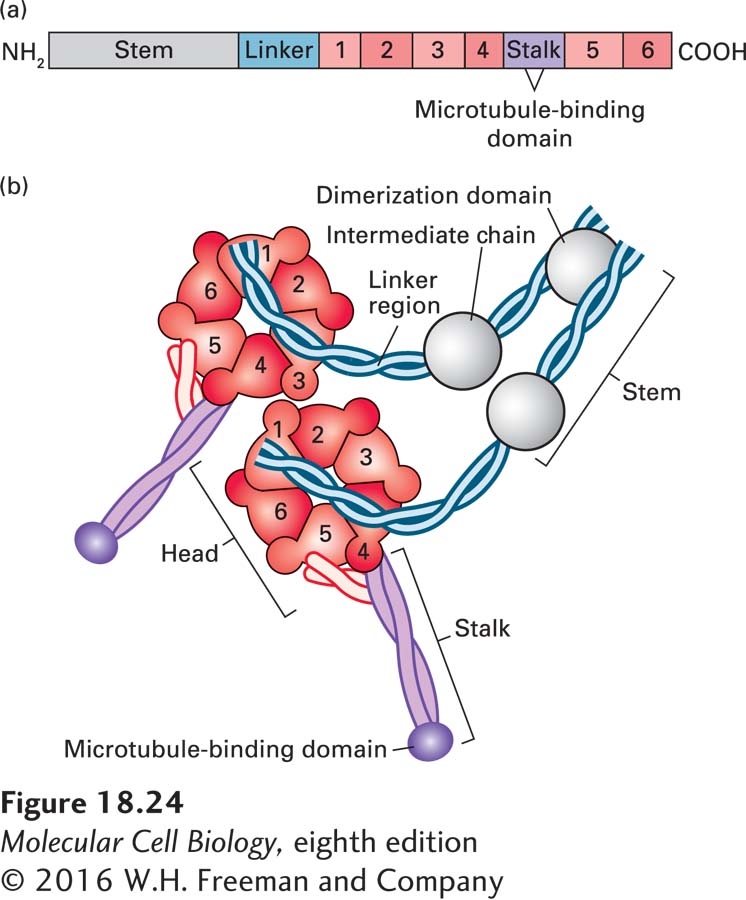
FIGURE 18-24 The domain structure of cytoplasmic dynein. (a) The dynein heavy chain, consisting of over 4000 amino acid residues, has several distinct domains. Following the stem and linker domains are six AAA repeats (peach, numbered 1–6), with the stalk and its microtubule-binding domain between repeats 4 and 5. The protein ends in an α-helical domain that supports the stalk. (b) The six AAA repeats assume a structure like petals on a flower. Emerging from this structure is a coiled-coil stalk domain with a microtubule-binding site at the end. A number of additional subunits associate with the stem region and can link dynein to cargo through dynactin. See R. D. Vale, 2003, Cell 112:467.
Electron microscopy, combined with recently acquired x-ray images of the structure of a dynein heavy chain, provides a glimpse of how dynein might work. The first AAA repeat is probably the only one involved in converting the hydrolysis of ATP into mechanical work. In the absence of a nucleotide, dynein binds to microtubules, and the linker is straight, lying across the AAA domain and associating with the first and fifth AAA repeats. Upon ATP binding, dynein dissociates from the microtubule, and the linker becomes bent to now cross between the second and third AAA repeats (“pre-stroke”; Figure 18-25a and b, left panels). Upon interaction with a microtubule and ATP hydrolysis and release of Pi, the linker straightens. This straightening is the power stroke that moves the cargo toward the (−) end of the microtubule (“post-stroke”; Figure 18-25a and b, right panels). The ADP is released, and the motor head remains bound to the microtubule. When ATP binds, the head is released, and the cycle repeats for another step.
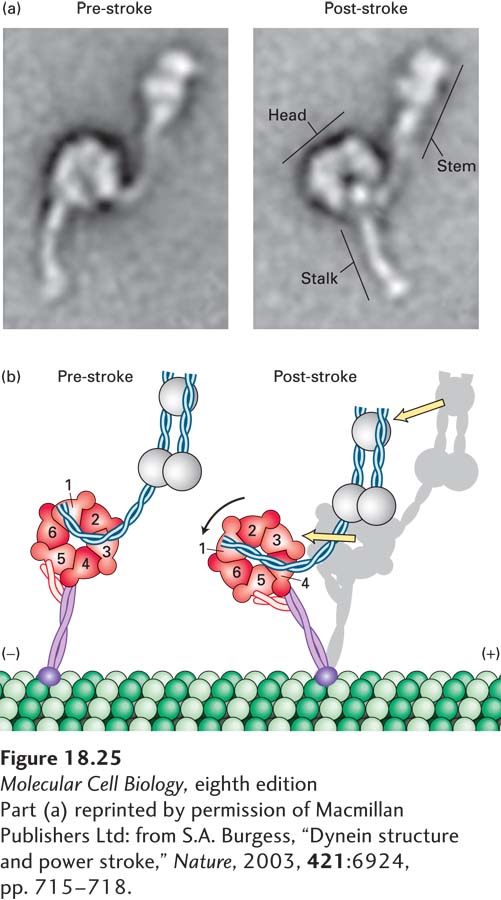
FIGURE 18-25 The power stroke of dynein. (a) Multiple images of purified single-headed dynein molecules in their pre-stroke and post-stroke states were recorded in an electron microscope and then averaged. The image at the left shows dynein in the ADP + Pi state, which represents the pre-stroke state, and the image at the right shows it in a nucleotide-free post-stroke state. (b) A comparison of the microscopic images combined with recently acquired structural data shows that the force-generation mechanism involves a change in the position of the linker, which causes a movement of the microtubule-binding stalk. See G. Bhabha et al., 2014, Cell 159:857.
[Part (a) reprinted by permission of Macmillan Publishers Ltd: from S.A. Burgess, “Dynein structure and power stroke,” Nature, 2003, 421:6924, pp. 715-718.
Unlike kinesin-1, cytoplasmic dynein cannot mediate cargo transport by itself. Rather, dynein-related transport requires regulators such as dynactin, a large protein complex that links dynein to its cargo and regulates its activity (Figure 18-26). Dynactin consists of 11 different types of subunits, functionally organized into two domains. One domain is built around one actin subunit and eight copies of the actin-related protein Arp1 assembled into a short filament. The end corresponding to the (+) end of this filament is capped by CapZ, the same capping protein that binds the (+) end of an actin filament (see Figure 17-12); a number of other subunits are associated with the (−) end. This Arp1-containing domain is responsible for binding cargo. The second domain of dynactin consists of a long protein called p150Glued, which contains the dynein-binding site and also has a microtubule-binding site at one end. Holding the two dynactin domains together is a protein called dynamitin—so named because when it is overexpressed, it dissociates (or “blows apart”) the two domains, making a nonfunctional complex. This feature has been very useful experimentally because it has allowed researchers to identify processes that are dependent on the interaction of dynein and dynactin, which are disrupted in cells overexpressing dynamitin.
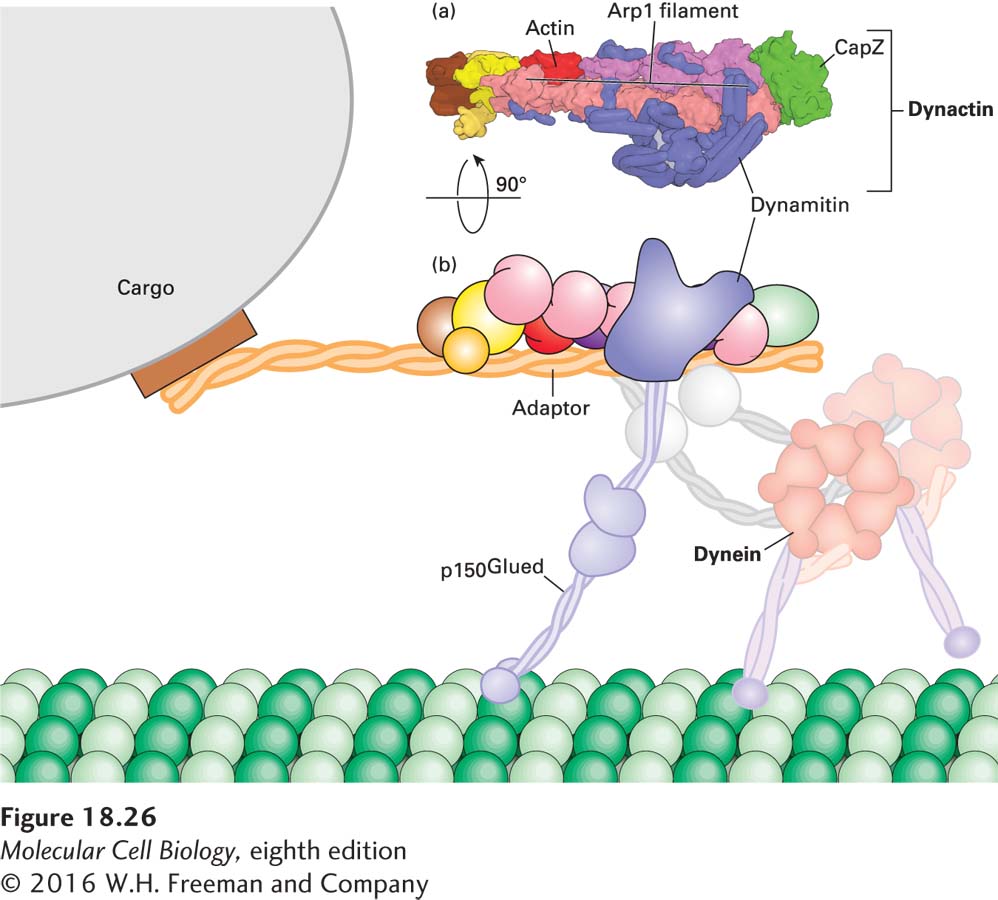
FIGURE 18-26 The dynactin complex links dynein to cargo. (a) One domain of the complex, which binds cargo, is built around a short filament made up of eight subunits of the actin-related protein Arp1 and one actin subunit, capped by CapZ. Another domain consists of the protein p150Glued, which has a microtubule-binding site on its distal end and is also involved in attaching cytoplasmic dynein to the complex. Dynamitin holds the two parts of the dynactin complex together. (b) Diagram of how the dynactin and dynein complex interact with each other and with a microtubule. See Urnavicius et al. 2015, Science 347:1441.
Recent work has shown that both dynein and dynactin can exist in an inactive form. However, when they encounter an appropriate cargo adapter, the three molecules form a tripartite complex in which dynactin is activated to bind microtubules and transport by dynein becomes highly processive, a critical property for transporting cargo over long distances (Figure 18-26b). In some circumstances, dynein must be transported in an inactive form to a specific location, where it is then activated. For example, it has been found that the dynactin p150Glued subunit binds EB1, allowing dynein to become associated with the growing (+) end of a microtubule. Why would dynein associate with the microtubule (+) end if it is a (−) end–directed motor? Recent work suggests that when dynein is associated with the (+) end of a microtubule via the dynactin-EB1 interaction, it is held in an inactive conformation. When the growing microtubule reaches the cell cortex, the inactive dynein and dynactin encounter an activator localized there. The dynein now becomes active, associating with the cortex and pulling on the microtubule that delivered it there! This mechanism has been shown to help orient the mitotic spindle in yeast, and it probably applies to other situations as well.
In addition to dynactin, other regulators of dynein exist. One group of proteins, two of which are LIS1 and NudE, is involved in regulating the activity of dynein during brain development. NudE links the dynein intermediate and light chains to LIS1 (Figure 18-27a). LIS1 then interacts with the ATPase domain of dynein to lengthen the duration of the power stroke, making the motor more processive under conditions of high load. Defects in LIS1 cause the fatal disease Miller-Dieker lissencephaly (from which the protein got its name); lissencephaly means “smooth brain,” as cortical folds and grooves are lacking in the brains of patients with this condition (Figure 18-27b). Mutations in LIS1 result in defects in both neuronal mitoses and neuronal migration from the ventricular zone to the cortical plate in early development, resulting in the smooth-brain phenotype and defects in mental development as well as many other abnormalities.
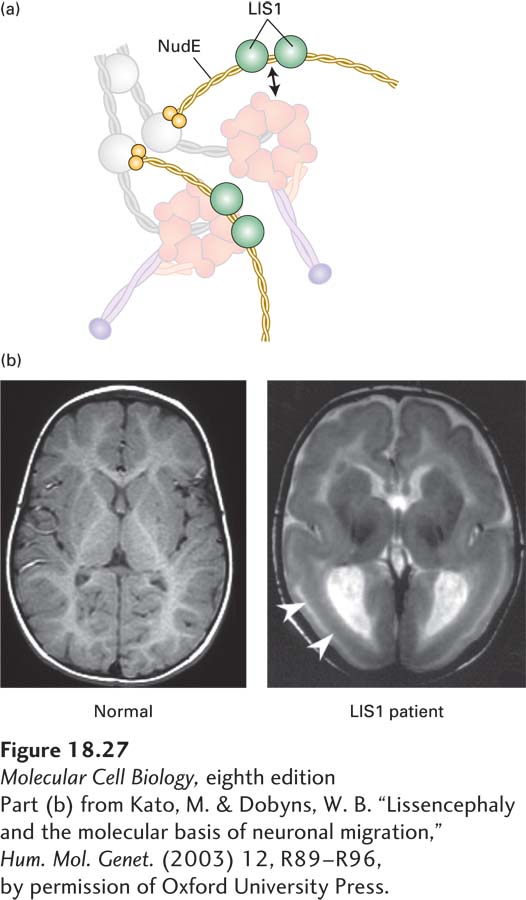
EXPERIMENTAL FIGURE 18-27 The LIS1 protein, which regulates dynein, is required for brain development. (a) Model of how NudE associates with dynein to allow LIS1 to interact with the ATPase domain of the dynein heavy chain. See McKenny et al., 2010, Cell 141:304–314. (b) Magnetic resonance images (MRIs) of a normal brain (left) and a brain from a patient with Miller-Dieker lissencephaly (right), which results from a lack of LIS1 function. Notice the absence of folding in the patient’s brain at the locations marked by the arrows.
[Part (b) from Kato, M. & Dobyns, W. B. “Lissencephaly and the molecular basis of neuronal migration,” Hum. Mol. Genet. (2003) 12, R89-R96, by permission of Oxford University Press.]



