Microtubule Dynamics Increase Dramatically in Mitosis
Although we have drawn static images of the stages of mitosis, microtubules in all stages of mitosis are highly dynamic. As we have seen, as cells enter mitosis, the ability of their centrosomes to nucleate the assembly of microtubules increases significantly (see Figure 18-36). In addition, microtubules become much more dynamic. How was this determined? In principle, one could label microtubules with a fluorescent tag and watch their individual behaviors, but practically speaking, there are too many microtubules in a mitotic spindle to follow. To get an average value for the dynamic instability of these microtubules, researchers can introduce fluorescently labeled tubulin into cells, which becomes incorporated randomly into all microtubules. They can then bleach the fluorescent label in a small region of the mitotic spindle and measure the rate at which fluorescence comes back using a technique known as fluorescence recovery after photobleaching (FRAP) (see Figure 4-23). Since the recovery of fluorescence is due to the assembly of new microtubules from soluble fluorescent tubulin dimers, its rate represents the average rate at which microtubules turn over. In a mitotic spindle, their half-life is about 15 seconds, whereas in an interphase cell, it is about 5 minutes. It should be noted that these are bulk measurements and that individual microtubules can be more stable or dynamic, as we will see.
What makes microtubules more dynamic in mitosis? As we discussed earlier, dynamic instability is a measure of relative contributions of growth rates, shrinkage rates, catastrophes, and rescues (see Figure 18-9). Analysis of microtubule dynamics in vivo shows that the enhanced instability of individual microtubules in mitosis is generated mainly by an increase in catastrophes and a decrease in rescues, with little change in rates of growth (i.e., lengthening) or shrinkage (i.e., shortening). Studies with extracts from frog oocytes have suggested that the main factor enhancing catastrophes in both interphase and mitotic extracts is depolymerization by kinesin-13 proteins. This can be seen in an in vitro assay in which microtubule assembly from pure tubulin is nucleated from purified centrosomes (Figure 18-39a). If kinesin-13 is added to the assay, many fewer microtubules are formed. However, if the protein XMAP215, which enhances assembly at the (+) end, is added with the kinesin-13, many microtubules are formed due to a dramatic reduction in catastrophe frequency. It turns out that the activity of kinesin-13 does not change significantly during the cell cycle, whereas the activity of XMAP215 is inhibited by its phosphorylation during mitosis (Figure 18-39b). This results in much more unstable (more dynamic) microtubules as the cell enters mitosis (Figure 18-39c).
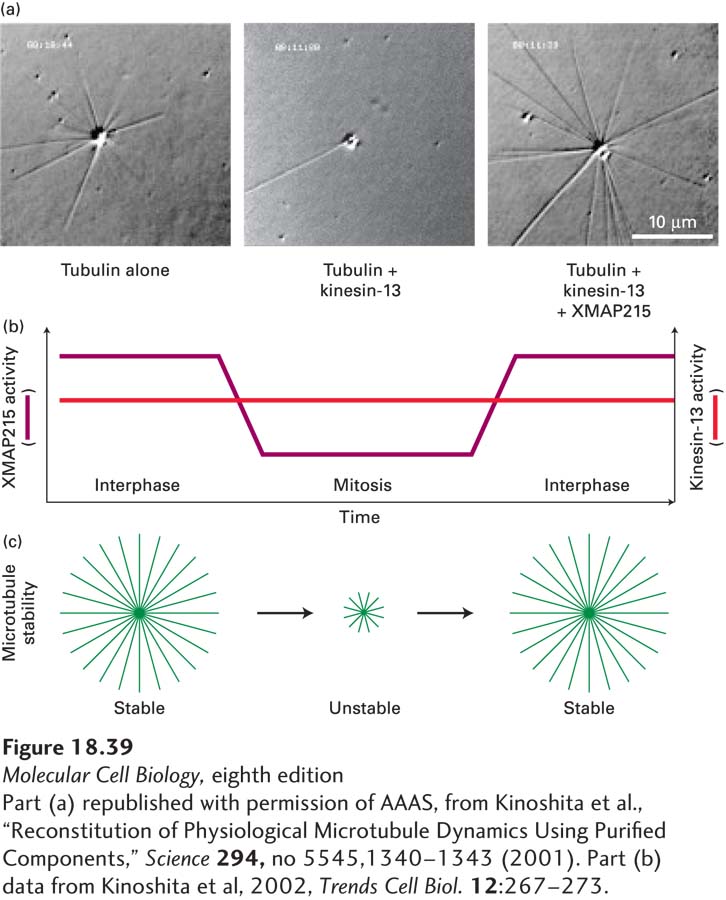
EXPERIMENTAL FIGURE 18-39 Microtubule dynamics increase in mitosis due to loss of a stabilizing MAP. (a) These three panels reveal the ability of centrosomes to assemble microtubules under various conditions: with pure tubulin (left); with tubulin and the destabilizing protein kinesin-13 (middle); and with tubulin, kinesin-13, and the stabilizing protein XMAP215 (Xenopus MAP of 215 kDa) (right). Further analysis shows that the major effect of XMAP215 is to suppress catastrophes induced by kinesin-13. (b) The increased dynamics of microtubules in mitosis is due to the inactivation of XMAP215 by phosphorylation. (c) Diagram comparing the stabilities of microtubules in interphase and in mitosis. Note that in addition to the decrease in stability in mitosis, the ability of MTOCs to nucleate microtubules increases dramatically in mitosis.
[Part (a) republished with permission of AAAS, from Kinoshita et al., “Reconstitution of Physiological Microtubule Dynamics Using Purified Components,” Science 294, no 5545,1340-1343 (2001). Part (b) data from Kinoshita et al, 2002, Trends Cell Biol. 12:267–273.]
