Individual Microtubules Exhibit Dynamic Instability
Early experiments revealed that most microtubules in animal cells disassemble when the cells are cooled to 4 °C and reassemble when the cells are rewarmed to 37 °C. Researchers realized that this intrinsic property of microtubules could be exploited to purify their components. Since brain tissue is rich in microtubules, soluble extracts of pig brains were prepared at 4 °C; these clarified extracts were then warmed to 37 °C to induce microtubule assembly. The assembled microtubules were collected into a pellet by centrifugation, separated from the supernatant, and then disassembled by adding buffer at 4 °C. After another cycle of assembly by warming, collection, and disassembly by cooling, researchers recovered microtubular protein, a collective term for αβ-tubulin and microtubule-associated proteins (MAPs). They were then able to fractionate the microtubular protein into pure αβ-tubulin and MAPs to study their behaviors separately. The investigators found that polymerization of dimeric αβ-tubulin into microtubules is greatly catalyzed by the presence of the MAPs.
Although a tremendous amount of research effort was devoted to characterizing the bulk polymerization properties of microtubular proteins in solution, its general relevance was superseded by subsequent studies examining the properties of individual microtubules. Nevertheless, some lessons learned from these earlier in vitro studies are important to microtubule biology. First, for assembly to occur, the αβ-tubulin concentration must be above the critical concentration (Cc), just as we saw for actin polymerization (see Figure 17-8). Second, at αβ-tubulin concentrations higher than Cc, dimers are added faster to one end of the microtubule than to the other (Figure 18-8). As with F-actin assembly, the preferred end for assembly, which is the end with β-tubulin exposed, is designated the (+) end. The (−) end has α-tubulin exposed (see Figure 18-3b).
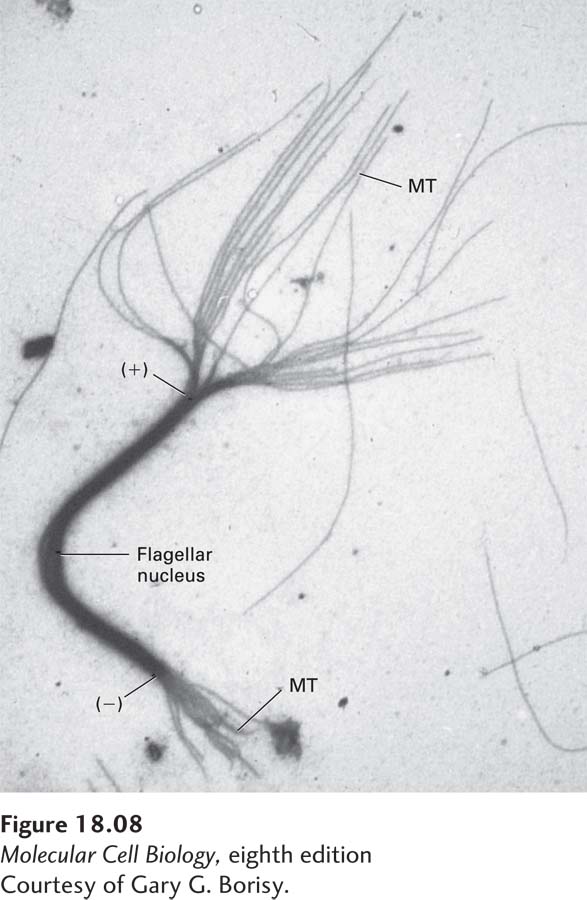
EXPERIMENTAL FIGURE 18-8 Microtubules grow preferentially at the (+) end. A fragment of a microtubule bundle from a flagellum was used as a nucleus for the in vitro addition of αβ-tubulin. The nucleating flagellar fragment is the thick bundle seen in this electron micrograph, with the newly formed microtubules (MT) radiating from its ends. The greater length of the microtubules at one end, the (+) end, indicates that tubulin subunits are added preferentially to this end.
[Courtesy of Gary G. Borisy.]
When studying the bulk properties of microtubule assembly, one might assume that all microtubules would behave similarly. However, when researchers examined the behavior of individual microtubules within a population, they found that this was not the case. Individual microtubule behavior was examined in a very simple experiment: microtubules were assembled in vitro and then sheared to break them into shorter pieces whose individual lengths could be analyzed by microscopy. Under these conditions, one might expect all the short microtubules either to grow or to shrink, depending on the free tubulin concentration. However, the investigators found that some of the microtubules grew in length, whereas others shortened very rapidly—thus indicating the existence of two distinct populations of microtubules. Further studies showed that individual microtubules could grow and then suddenly experience a catastrophe: an abrupt transition to a shrinking phase during which the microtubule would undergo rapid depolymerization. Moreover, sometimes a depolymerizing microtubule end could go through a rescue and begin growing again (Figure 18-9). Although this phenomenon was first seen in vitro, analysis of fluorescently labeled tubulin microinjected into live cells showed that microtubules in cells also undergo periods of growth and shrinkage (Figure 18-10). This alternation between growing and shrinking states is known as dynamic instability. Thus the dynamic life of a microtubule end is determined by the rate of growth, the frequency of catastrophes, the rate of depolymerization, and the frequency of rescues. As we will see later, these features of microtubule dynamics are controlled in vivo. Since the (−) ends of microtubules in animal cells are generally anchored to an MTOC, this dynamism is most relevant to the (+) end of the microtubule.
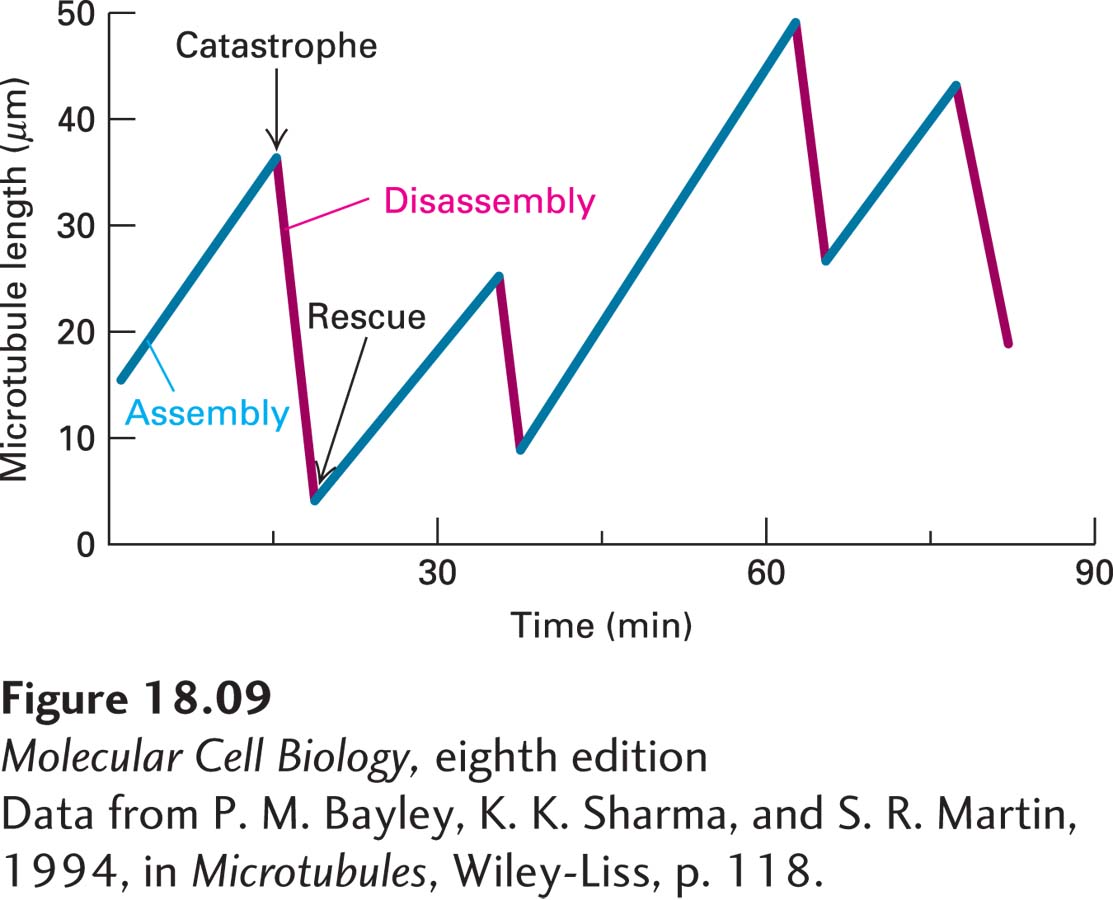
FIGURE 18-9 Dynamic instability of microtubules in vitro. Individual microtubules can be observed in the light microscope and their lengths plotted at different times during assembly and disassembly. Assembly and disassembly each proceed at uniform rates, but there is a big difference between the rate of assembly and that of disassembly, as seen in the different slopes of the lines. Shortening of a microtubule is much more rapid (7 µm/min) than growth (1 µm/min). Notice the abrupt transitions to the shrinkage stage (catastrophe) and to the elongation stage (rescue).
[Data from P. M. Bayley, K. K. Sharma, and S. R. Martin, 1994, in Microtubules, Wiley-Liss, p. 118.]
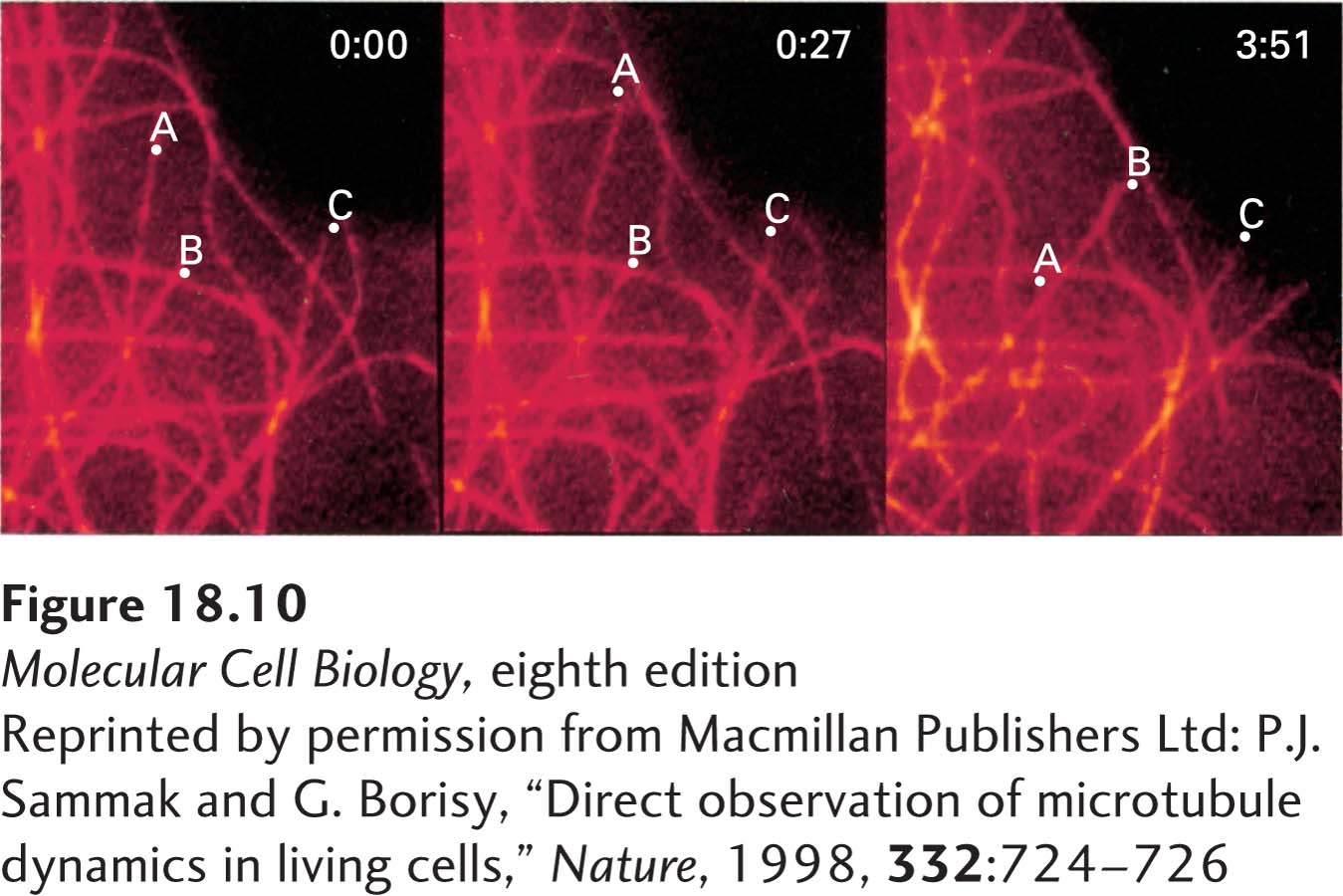
EXPERIMENTAL FIGURE 18-10 Fluorescence microscopy reveals growth and shrinkage of individual microtubules in vivo. Fluorescently labeled tubulin was microinjected into cultured human fibroblasts. The cells were chilled to depolymerize preexisting microtubules into tubulin dimers and were then incubated at 37 °C to allow repolymerization, which incorporated the fluorescent tubulin into all the cells’ microtubules. A region of a cell periphery was viewed in the fluorescence microscope at 0 seconds, 27 seconds later, and 3 minutes 51 seconds later (left to right panels). During this period, several microtubules can be seen to have elongated and shortened. The dots labeled A, B, and C mark the positions of the ends of three microtubules.
[Reprinted by permission from Macmillan Publishers Ltd: P.J. Sammak and G. Borisy, “Direct observation of microtubule dynamics in living cells,” Nature, 1998, 332:724-726.]
What is the molecular basis of dynamic instability? If you look carefully at the ends of growing and shrinking microtubules in the electron microscope, you can see that they are quite different. A growing microtubule has a relatively blunt end, whereas a depolymerizing end has protofilaments peeling off like rams’ horns (Figure 18-11). In fact, the growing microtubule end is not simply a blunt end, but rather a short and gently curved sheet-like structure, formed by the addition of tubulin dimers to the ends of protofilaments, that then rolls up along the seam to form the cylindrical microtubule with straight protofilaments.
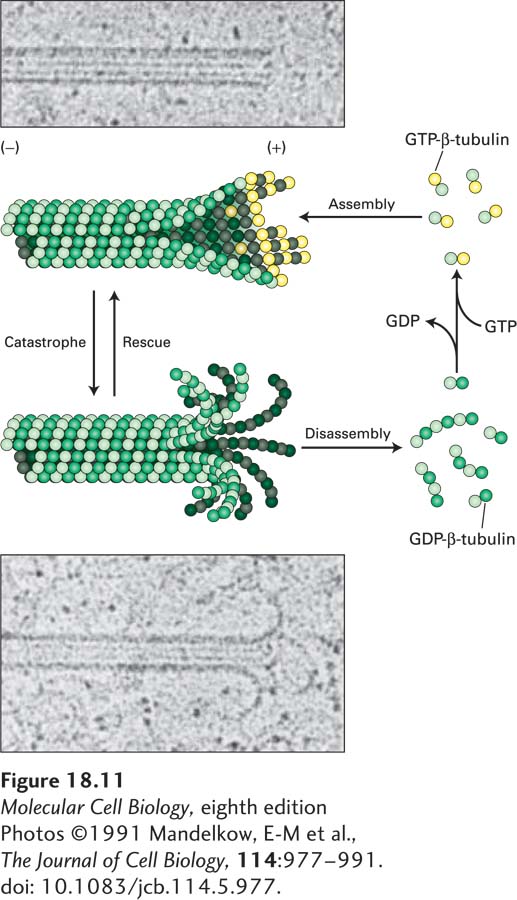
FIGURE 18-11 Dynamic instability depends on the presence or absence of a GTP-β-tubulin cap. Images taken in the electron microscope of frozen samples of a growing microtubule (upper) and a shrinking microtubule (lower). Notice that the end of the growing microtubule has a blunter end, whereas the end of the shrinking one has curls like a ram’s horns. As the diagram shows, a microtubule with GTP-β-tubulin on the end of each protofilament is strongly favored to grow. However, a microtubule with GDP-β-tubulin at the ends of the protofilaments forms a curved structure and will undergo rapid disassembly. Switches between growing and shrinking phases, called rescues and catastrophes, can occur, and the rate of switching is regulated by associated proteins. See A. Desai and T. J. Mitchison, 1997, Annu. Rev. Cell Dev. Bi. 13:83–117.
[Photos ©1991 Mandelkow, E-M et al., The Journal of Cell Biology, 114:977–991. doi: 10.1083/jcb.114.5.977.]
A simple structural difference accounts for the morphology of these two different classes of microtubule (+) ends. As we noted above, the β-subunit of the αβ-tubulin dimer is exposed on the (+) end of each protofilament. Using a GDP analog, researchers have found that artificially made single protofilaments—which are not exposed to lateral interactions—made up of repeating αβ-tubulin dimers containing GDP-β-tubulin are curved, like a ram’s horn. However, artificially made single protofilaments made up of αβ-tubulin dimers in which β-tubulin has a bound GTP analog are only slightly curved. The assembling end of a microtubule contains these slightly curved nascent protofilaments, giving rise to the gently curved sheets and single protofilaments characteristic of growing ends (see Figure 18-11). The gently curved protofilaments containing GTP-β-tubulin then zip up by lateral interactions to form straight protofilaments within the microtubule. By contrast, shrinking microtubules with curled ends terminate in GDP-β-tubulin. Therefore, if the GTP molecules in the terminal β-tubulins become hydrolyzed on a microtubule that has stopped growing, a formerly blunt-ended microtubule will curl and a catastrophe will ensue. These relationships are summarized in Figure 18-11.
These results have an additional and fascinating implication, but to understand it we must consider the growing microtubule in more detail. The addition of a dimer to the (+) end of a protofilament on a growing microtubule involves an interaction between the preexisting terminal β-subunit and the new α-subunit. This interaction enhances the hydrolysis of the GTP in the formerly terminal β-subunit to GDP and Pi. The inorganic phosphate is then released to yield a microtubule with predominantly GDP-β-tubulin down its length. However, the β-tubulin in the newly added dimer contains GTP. Thus each protofilament in a growing microtubule has mostly GDP-β-tubulin down its length and is capped by a few terminal dimers containing GTP-β-tubulin and GDP-Pi-β-tubulin. As we mentioned above, an isolated protofilament containing GDP-β-tubulin is curved along its length, so when it is present in a microtubule, why doesn’t it break out and peel away? The lateral protofilament-protofilament interactions in the GTP-β-tubulin cap are sufficiently strong that they do not allow the microtubule to unpeel at its end—and so the protofilaments behind the GTP-β-tubulin cap are constrained from unpeeling (see Figure 18-11). The energy released by GTP hydrolysis in the subunits behind the cap is stored within the lattice as structural strain waiting to be released when the GTP-β-tubulin cap is lost. If the GTP-β-tubulin cap is lost, the stored energy can do work if some structure, such as a chromosome, is attached to the disassembling microtubule end. As we will see, this stored energy contributes to the movement of chromosomes during the anaphase stage of mitosis.
How can a disassembling microtubule suddenly be rescued to grow again? A possible answer to this perplexing problem has recently been suggested. Using an antibody that recognizes only GTP-β-tubulin and not GDP-β-tubulin, researchers have found that “islands” of GTP-β-tubulin can persist along the length of an assembled microtubule. It seems likely that when a disassembling microtubule encounters one of these GTP-β-tubulin islands, disassembly pauses, and a rescue may be provoked.



