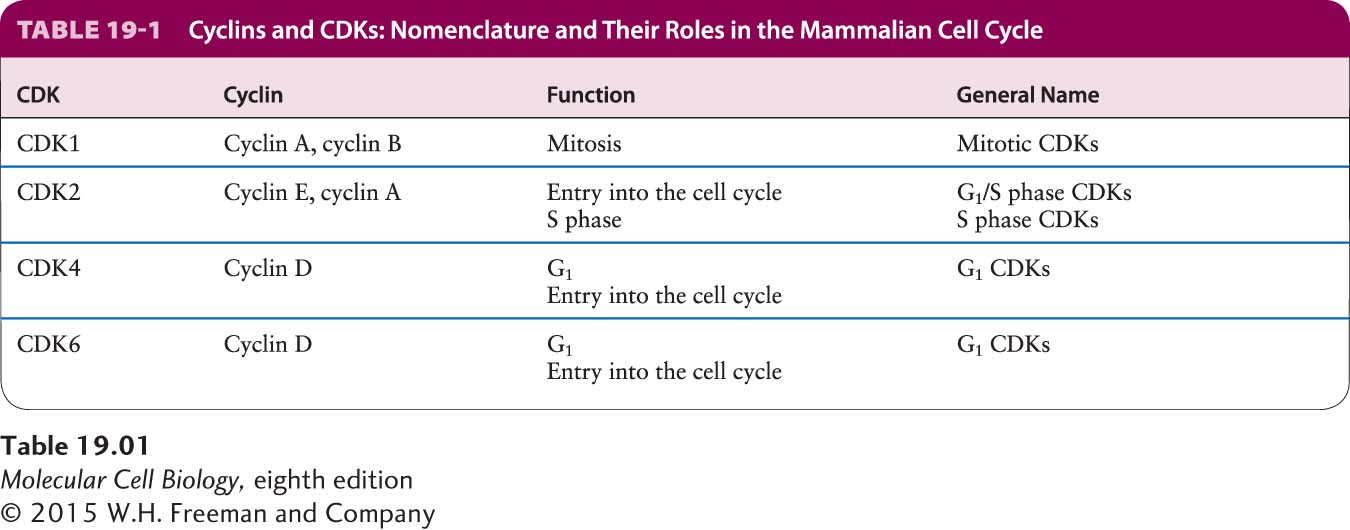
Cyclin-Dependent Kinases Are Small Protein Kinases That Require a Regulatory Cyclin Subunit for Their Activity
Cyclin-dependent kinases are a family of small (30–40 kD) serine/threonine kinases. They are not active in the monomeric form, but require an activating subunit to be active as protein kinases. In budding and fission yeasts, a single CDK controls progression through the cell cycle. Its activity is specified by cell-cycle-stage-specific cyclin subunits. Mammalian cells contain as many as nine CDKs, with four of them, CDK1, CDK2, CDK4, and CDK6, having clearly been shown to regulate cell cycle progression. They bind to different types of cyclins and, together with those cyclins, promote different cell cycle transitions. CDK4 and CDK6 are G1 CDKs that promote entry into the cell cycle, CDK2 functions as a G1/S phase and S phase CDK, and CDK1 is the mitotic CDK. For historical reasons, the names of various cyclin-dependent kinases from yeasts and vertebrates differ. Whenever possible, we will use the general terms G1, G1/S phase, S phase, and mitotic CDKs to describe CDKs instead of the species-specific terminology. Table 19-1 lists the different names of the various CDKs and indicates when in the cell cycle they are active.
CDKs are regulated not only by cyclin binding, but also by both activating and inhibitory phosphorylation. Together, these regulatory events ensure that CDKs are active only at the appropriate cell cycle stage. The three-dimensional structure of CDKs provides insight into how the activity of these protein kinases is regulated. Nonphosphorylated, inactive CDK contains a flexible region, called the T loop or activation loop, that is highly conserved among protein kinases and is discussed extensively in Chapter 16. This loop blocks access of protein substrates to the active site where ATP is bound (Figure 19-11a), explaining why free CDK, unbound to cyclin, has little protein kinase activity. Nonphosphorylated CDK bound to one of its cyclin partners has minimal but detectable protein kinase activity in vitro, although it may be essentially inactive in vivo. Extensive interactions between the cyclin and the T loop cause a dramatic shift in the position of the T loop, thereby exposing the CDK active site (Figure 19-11b). As we will see shortly, high activity of the cyclin-CDK complex requires phosphorylation of the activating threonine in the T loop, causing additional conformational changes in the cyclin-CDK complex that greatly increase its affinity for protein substrates (Figure 19-11c). As a result, the kinase activity of the phosphorylated complex is a hundredfold greater than that of the nonphosphorylated complex.
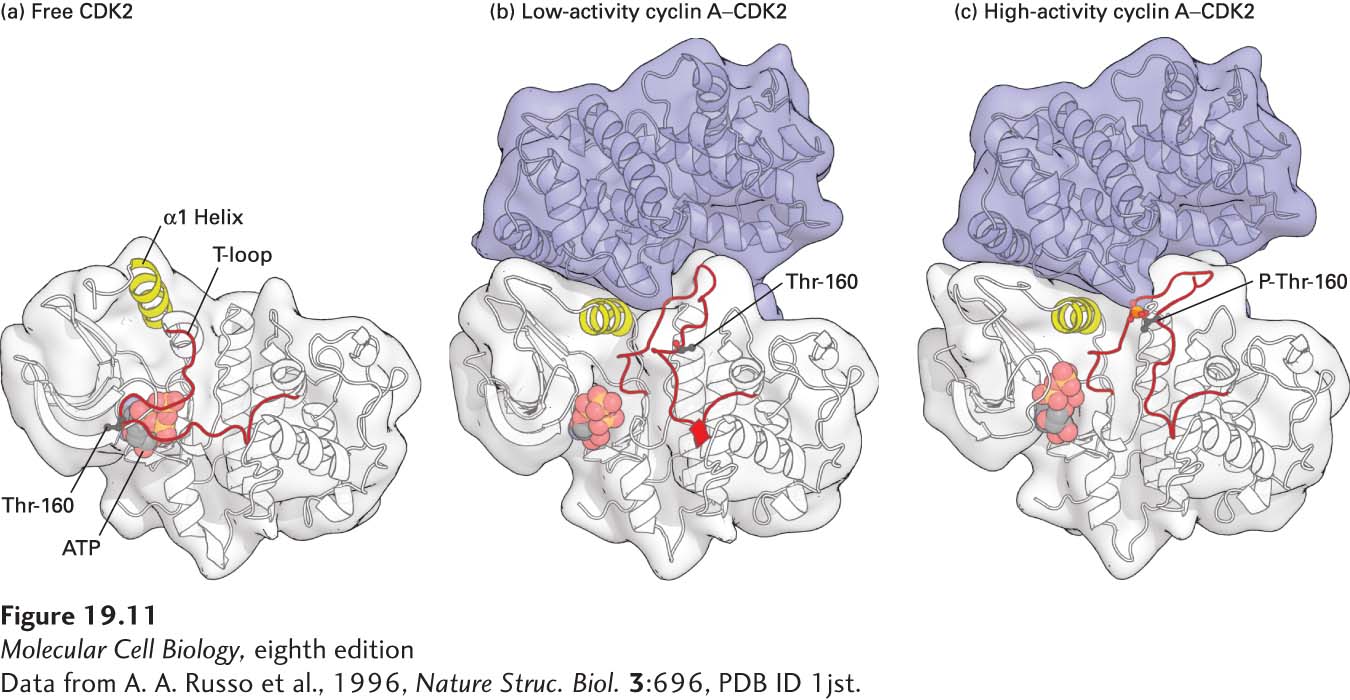
FIGURE 19-11 Structural models of human CDK2. (a) Free, inactive CDK2 not bound to its cyclin subunit, cyclin A. In free CDK2, the T loop blocks access of protein substrates to the γ phosphate of the bound ATP, shown as a ball-and-stick model. The conformations of the T-loop and the region highlighted in yellow (α1 Helix) are altered when CDK is bound to cyclin A. (b) Nonphosphorylated, low-activity cyclin A–CDK2 complex. Conformational changes induced by binding of a domain of cyclin A (blue) cause the T loop to pull away from the active site of CDK2 so that substrate proteins can bind. The α1 helix in CDK2, which interacts extensively with cyclin A, moves several angstroms into the catalytic cleft, repositioning key catalytic side chains required for the phosphotransfer reaction. The black ball marks the position of the threonine (Thr-160) whose phosphorylation activates CDKs. (c) Phosphorylated, high-activity cyclin A–CDK2 complex. The conformational changes induced by phosphorylation of the activating threonine (red ball) alter the shape of the substrate-binding surface, greatly increasing the affinity for protein substrates. See P. D. Jeffrey et al., 1995, Nature 1995, 376:313-20.
[Data from A. A. Russo et al., 1996, Nature Struc. Biol. 3:696, PDB ID 1jst.]

