Cyclin Levels Are Primarily Regulated by Protein Degradation
Multiple mechanisms ensure that CDKs are active at the right stage of the cell cycle. Table 19-2 lists the key regulators of CDKs. The timely activation of CDKs depends, in part, on the presence of the appropriate cyclins in the cell cycle stage at which they are needed. In this section, we discuss how the regulation of cyclin levels is brought about. Transcriptional control of the cyclin subunits is one mechanism that ensures proper temporal expression of the cyclins. In somatic cells and yeast, waves of transcription factor activity help establish waves of cyclin activity. A general principle here is that an earlier wave of transcriptional activity helps produce the factors essential to generate a subsequent transcriptional wave. As we will see in Section 19.4, transcription of the G1/S phase cyclins is promoted by the E2F transcription factor complex. Among the many other genes whose transcription E2F promotes are those encoding the transcription factors that promote the synthesis of mitotic cyclins.
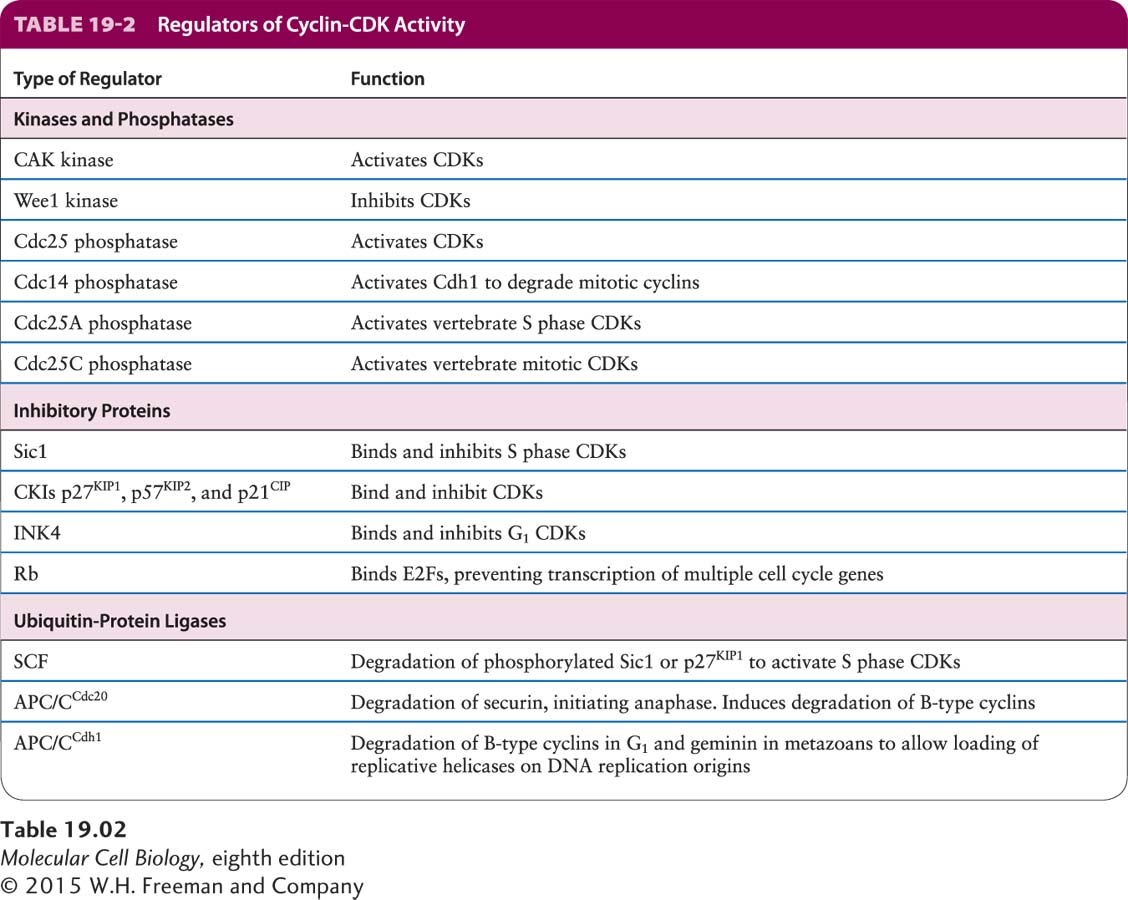
The most important regulatory control that restricts cyclins to the appropriate cell cycle stage is ubiquitin-mediated, proteasome-dependent protein degradation. Because protein degradation is an irreversible process, in the sense that the protein can be replenished only through de novo protein synthesis, this regulatory mechanism is ideal to ensure that the cell cycle engine is driven forward and that cells cannot “go backward” in the cell cycle. In other words, once a particular cyclin is degraded, the processes that it activated can no longer take place.
Recall that during ubiquitin-mediated protein degradation, ubiquitin-protein ligases ubiquitinylate substrate proteins, marking them for degradation by proteasomes (see Figure 3-31). Cyclins are degraded through the action of two different ubiquitin-protein ligases, SCF (named after the first letters of its constituents, Skp1, Cullin, and F-box proteins) and the anaphase-promoting complex or cyclosome (abbreviated as APC/C in this chapter). SCF controls the G1–S phase transition by degrading G1/S phase cyclins and, as we will see in detail shortly, CDK inhibitory proteins. APC/C degrades S phase and mitotic cyclins, thereby promoting the exit from mitosis. APC/C also controls the onset of chromosome segregation at the metaphase-anaphase transition by degrading an anaphase inhibitory protein (as discussed in Section 19.6).
SCF and APC/C are multisubunit ubiquitin-protein ligases that belong to the RING finger family of ubiquitin-protein ligases. Despite the fact that SCF and APC/C belong to the same ubiquitin-protein ligase family, their regulation is quite different. SCF recognizes its substrates only when they are phosphorylated. It is continuously active throughout the cell cycle, and cell-cycle-regulated phosphorylation of its substrates ensures that they are degraded only at certain stages of the cell cycle. In the case of APC/C-dependent protein degradation, the regulation is reversed: substrates are recognizable throughout the cell cycle, but the activity of APC/C is regulated. APC/C is activated by phosphorylation at the metaphase-anaphase transition through the action of mitotic CDKs and other protein kinases. APC/C is then active throughout the rest of mitosis and during G1 to promote the degradation of cyclins and other mitotic regulators (see Figure 19-10). The substrate specificity of active, phosphorylated APC/C is determined in part by its association with one of two related substrate targeting factors called Cdc20 and Cdh1. During anaphase, APC/C bound to Cdc20 ubiquitinylates proteins that inhibit chromosome segregation, while during telophase and G1, APC/C bound to Cdh1 targets different substrates for degradation.
The substrates of APC/C contain recognition motifs. The first to be identified was the destruction box. It is found in most S phase and mitotic cyclins and is both necessary and sufficient to target proteins for degradation. The importance of cyclin degradation was again first demonstrated for mitotic cyclins. Deletion of the destruction box in mitotic cyclins prevented cells from exiting mitosis, demonstrating that mitotic exit requires the degradation of mitotic cyclin (see Classic Experiment 19-2). Later studies showed that inhibiting the degradation of other cyclins also severely affected cell cycle progression, indicating that ubiquitin-mediated degradation of cyclins is an essential aspect of the eukaryotic cell cycle.
